
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned".
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This involves vegetable or animal fats and oils being reacted with short-chain alcohols. The alcohols used should be of low molecular weight. Ethanol is the most used because of its low cost, however, greater conversions into biodiesel can be reached using methanol. Although the transesterification reaction can be catalyzed by either acids or bases, the base-catalyzed reaction is more common. This path has lower reaction times and catalyst cost than those acid catalysis. However, alkaline catalysis has the disadvantage of high sensitivity to both water and free fatty acids present in the oils. August 10 is international biodiesel day
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

An ionic liquid (IL) is a salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.

Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annually. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.
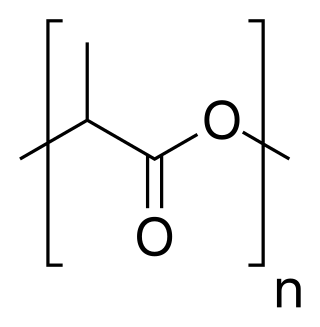
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid-liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
Deep eutectic solvents or DESs are solutions of Lewis or Brønsted acids and bases which form a eutectic mixture. Deep eutectic solvents are highly tunable through varying the structure or relative ratio of parent components and thus have a wide variety of potential applications including catalytic, separation, and electrochemical processes. The parent components of deep eutectic solvents engage in a complex hydrogen bonding network which results in significant freezing point depression as compared to the parent compounds. The extent of freezing point depression observed in DESs is well illustrated by a mixture of choline chloride and urea in a 1:2 mole ratio. Choline chloride and urea are both solids at room temperature with melting points of 302 °C and 133 °C respectively, yet the combination of the two in a 1:2 molar ratio forms a liquid with a freezing point of 12 °C. DESs share similar properties to ionic liquids such as tunability and lack of flammability yet are distinct in that ionic liquids are neat salts composed exclusively of discrete ions. In contrast to ordinary solvents, such as Volatile Organic Compounds (VOC), DESs are non-flammable, and possess low vapour pressures and toxicity.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids.

γ-Valerolactone (GVL) or gamma-valerolactone is an organic compound with the formula C5H8O2. This colourless liquid is one of the more common lactones. GVL is chiral but is usually used as the racemate. It is readily obtained from cellulosic biomass and is a potential fuel and green solvent.

1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
11-Aminoundecanoic acid is an organic compound with the formula H2N(CH2)10CO2H. This white solid is classified as an amine and a fatty acid. 11-Aminoundecanoic acid is a precursor to Nylon-11.

Sucrose esters or sucrose fatty acid esters are a group of non-naturally occurring surfactants chemically synthesized from the esterification of sucrose and fatty acids. This group of substances is remarkable for the wide range of hydrophilic-lipophilic balance (HLB) that it covers. The polar sucrose moiety serves as a hydrophilic end of the molecule, while the long fatty acid chain serves as a lipophilic end of the molecule. Due to this amphipathic property, sucrose esters act as emulsifiers; i.e., they have the ability to bind both water and oil simultaneously. Depending on the HLB value, some can be used as water-in-oil emulsifiers, and some as oil-in-water emulsifiers. Sucrose esters are used in cosmetics, food preservatives, food additives, and other products. A class of sucrose esters with highly substituted hydroxyl groups, olestra, is also used as a fat replacer in food.
Mineral processing and extraction of metals are very energy-intensive processes, which are not exempted of producing large volumes of solid residues and wastewater, which also require energy to be further treated and disposed. Moreover, as the demand for metals increases, the metallurgical industry must rely on sources of materials with lower metal contents both from a primary and/or secondary raw materials. Consequently, mining activities and waste recycling must evolve towards the development of more selective, efficient and environmentally friendly mineral and metal processing routes.




























