Histology, also known as microscopic anatomy or microanatomy, is the branch of biology that studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into organology, the study of organs, histology, the study of tissues, and cytology, the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms.

Eosin is the name of several fluorescent acidic compounds which bind to and form salts with basic, or eosinophilic, compounds like proteins containing amino acid residues such as arginine and lysine, and stains them dark red or pink as a result of the actions of bromine on eosin. In addition to staining proteins in the cytoplasm, it can be used to stain collagen and muscle fibers for examination under the microscope. Structures that stain readily with eosin are termed eosinophilic. In the field of histology, Eosin Y is the form of eosin used most often as a histologic stain.

Alizarin is an organic compound with formula C
14H
8O
4 that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural dye to be produced synthetically.

Romanowsky staining is a prototypical staining technique that was the forerunner of several distinct but similar stains widely used in hematology and cytopathology. Romanowsky-type stains are used to differentiate cells for microscopic examination in pathological specimens, especially blood and bone marrow films, and to detect parasites such as malaria within the blood.

A mordant or dye fixative is a substance used to set dyes on fabrics. It does this by forming a coordination complex with the dye, which then attaches to the fabric. It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. Although mordants are still used, especially by small batch dyers, they have been largely displaced in industry by directs.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Haematoxylum campechianum is a species of flowering tree in the legume family, Fabaceae, that is native to southern Mexico,where it is known as Árbol de campeche, and introduced to the Caribbean, northern Central America, and other localities around the world. The tree was of great economic importance from the 17th century to the 19th century, when it was commonly logged and exported to Europe for use in dyeing fabrics. The modern nation of Belize developed from 17th- and 18th-century logging camps established by the English. The tree's scientific name means "bloodwood".

Eosinophilic is the staining of tissues, cells, or organelles after they have been washed with eosin, a dye.

Basophilic is a technical term used by pathologists. It describes the appearance of cells, tissues and cellular structures as seen through the microscope after a histological section has been stained with a basic dye. The most common such dye is haematoxylin.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.
Trichrome staining is a histological staining method that uses two or more acid dyes in conjunction with a polyacid. Staining differentiates tissues by tinting them in contrasting colours. It increases the contrast of microscopic features in cells and tissues, which makes them easier to see when viewed through a microscope.

Eosin Y, also called C.I. 45380 or C.I. Acid Red 87, is a member of the triarylmethane dyes. It is produced from fluorescein by bromination.

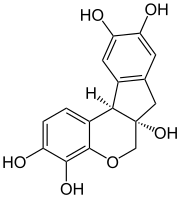
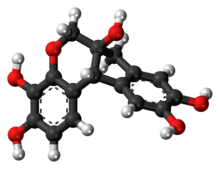
Hematein or haematein is an oxidized derivative of haematoxylin, used in staining. Haematein should not be confused with haematin, which is a brown to black iron-containing pigment formed by decomposition of haemoglobin. In the Colour Index, haematein is called haematine.

Papanicolaou stain is a multichromatic (multicolored) cytological staining technique developed by George Papanicolaou in 1942. The Papanicolaou stain is one of the most widely used stains in cytology, where it is used to aid pathologists in making a diagnosis. Although most notable for its use in the detection of cervical cancer in the Pap test or Pap smear, it is also used to stain non-gynecological specimen preparations from a variety of bodily secretions and from small needle biopsies of organs and tissues. Papanicolaou published three formulations of this stain in 1942, 1954, and 1960.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.

Phosphotungstic acid haematoxylin (PTAH) is a mix of haematoxylin with phosphotungstic acid, used in histology for staining.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).

Brazilin is a naturally occurring, a homoisoflavonoid, red dye obtained from the wood of Paubrasilia echinata, Biancaea sappan, Caesalpinia violacea, and Haematoxylum brasiletto. Brazilin has been used since at least the Middle Ages to dye fabric, and has been used to make paints and inks as well. The specific color produced by the pigment depends on its manner of preparation: in an acidic solution brazilin will appear yellow, but in an alkaline preparation it will appear red. Brazilin is closely related to the blue-black dye precursor hematoxylin, having one fewer hydroxyl group. Brazilein, the active dye agent, is an oxidized form of brazilin.
The von Kossa histological stain is used to quantify mineralization in cell culture and histological sections.

Dmitri Leonidovich Romanowsky was a Russian physician who is best known for his invention of an eponymous histological stain called Romanowsky stain. It paved the way for the discovery and diagnosis of microscopic pathogens, such as malarial parasites, and later developments of new histological stains that became fundamental to microbiology and physiology.