
The neutron is a subatomic particle, symbol
n
or
n0
, which has a neutral charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics.
Neutronium is a hypothetical substance composed purely of neutrons. The word was coined by scientist Andreas von Antropoff in 1926 for the hypothetical "element of atomic number zero" that he placed at the head of the periodic table. However, the meaning of the term has changed over time, and from the last half of the 20th century onward it has been also used to refer to extremely dense substances resembling the neutron-degenerate matter theorized to exist in the cores of neutron stars; hereinafter "degenerate neutronium" will refer to this.

In chemistry and physics, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines an isotope's mass number.
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing about 75% hydrogen and 24% helium by mass. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium remains small, so that the universe still has approximately the same composition.

In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "magic numbers" of protons and neutrons in the superheavy mass region.
A tetraneutron is a hypothetical stable cluster of four neutrons. The existence of this cluster of particles is not supported by current models of nuclear forces. There is some empirical evidence suggesting that this particle does exist, based on a 2001 experiment by Francisco-Miguel Marqués and co-workers at the Ganil accelerator in Caen using a novel detection method in observations of the disintegration of beryllium and lithium nuclei. However, subsequent attempts to replicate this observation have failed.
The term p-process is used in two ways in the scientific literature concerning the astrophysical origin of the elements (nucleosynthesis). Originally it referred to a proton capture process which is the source of certain, naturally occurring, neutron-deficient isotopes of the elements from selenium to mercury. These nuclides are called p-nuclei and their origin is still not completely understood. Although it was shown that the originally suggested process cannot produce the p-nuclei, later on the term p-process was sometimes used to generally refer to any nucleosynthesis process supposed to be responsible for the p-nuclei.

In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126. For protons, this corresponds to the elements helium, oxygen, calcium, nickel, tin, lead and the hypothetical unbihexium, although 126 is so far only known to be a magic number for neutrons. Atomic nuclei consisting of such a magic number of nucleons have a higher average binding energy per nucleon than one would expect based upon predictions such as the semi-empirical mass formula and are hence more stable against nuclear decay.

Hydrogen (1H) has three naturally occurring isotopes, sometimes denoted 1H, 2H, and 3H. The first two of these are stable, while 3H has a half-life of 12.32 years. There are also heavier isotopes, which are all synthetic and have a half-life less than one zeptosecond. Of these, 5H is the most stable, and 7H is the least.
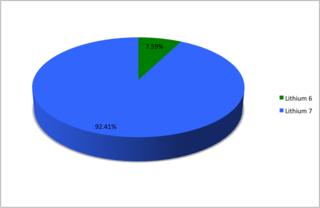
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant: about 92.5 percent of the atoms. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon when compared with the adjacent lighter and heavier elements, helium (~7.1 MeV) and beryllium (~6.5 MeV). The longest-lived radioisotope of lithium is lithium-8, which has a half-life of just 839.4 milliseconds. Lithium-9 has a half-life of 178.3 milliseconds, and lithium-11 has a half-life of about 8.75 milliseconds. All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is lithium-4, which decays by proton emission with a half-life of about 9.1×10−23 seconds, although the half-life of lithium-3 is yet to be determined, and is likely to be much shorter, like helium-2 (diproton) which undergoes proton emission within 10−9 s.
Although there are nine known isotopes of helium (2He), only helium-3 and helium-4 are stable. All radioisotopes are short-lived, the longest-lived being 6
He
with a half-life of 806.7 milliseconds. The least stable is 5
He
, with a half-life of 7.6×10−22 s, although it is possible that 2
He
has an even shorter half-life.
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999. Flerovium has seven known isotopes, and possibly 2 nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but the unconfirmed 290Fl may have a longer half-life of 19 seconds.

In nuclear physics, the valley of stability is a characterization of the stability of nuclides to radioactivity based on their binding energy. Nuclides are composed of protons and neutrons. The shape of the valley refers to the profile of binding energy as a function of the numbers of neutrons and protons, with the lowest part of the valley corresponding to the region of most stable nuclei. The line of stable nuclides down the center of the valley of stability is known as the line of beta stability. The sides of the valley correspond to increasing instability to beta decay. The decay of a nuclide becomes more energetically favorable the further it is from the line of beta stability. The boundaries of the valley correspond to the nuclear drip lines, where nuclides become so unstable they emit single protons or single neutrons. Regions of instability within the valley at high atomic number also include radioactive decay by alpha radiation or spontaneous fission. The shape of the valley is roughly an elongated paraboloid corresponding to the nuclide binding energies as a function of neutron and atomic numbers.

Beryllium-8 is a radionuclide with 4 neutrons and 4 protons. It is an unbound resonance and nominally an isotope of beryllium. It decays into two alpha particles with a half-life on the order of 10−16 seconds; this has important ramifications in stellar nucleosynthesis as it creates a bottleneck in the creation of heavier chemical elements. The properties of 8Be have also led to speculation on the fine tuning of the Universe, and theoretical investigations on cosmological evolution had 8Be been stable.

The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively-charged nucleus, with a cloud of negatively-charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.
The EMC effect is the surprising observation that the cross section for deep inelastic scattering from an atomic nucleus is different from that of the same number of free protons and neutrons. From this observation, it can be inferred that the quark momentum distributions in nucleons bound inside nuclei are different from those of free nucleons. This effect was first observed in 1983 at CERN by the European Muon Collaboration, hence the name "EMC effect". It was unexpected, since the average binding energy of protons and neutrons inside nuclei is insignificant when compared to the energy transferred in deep inelastic scattering reactions that probe quark distributions. While over 1000 scientific papers have been written on the topic and numerous hypotheses have been proposed, no definitive explanation for the cause of the effect has been confirmed. Determining the origin of the EMC effect is one of the major unsolved problems in the field of nuclear physics.

The nuclear drip line is the boundary delimiting the zone beyond which atomic nuclei decay by the emission of a proton or neutron.
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be precisely measured with atomic spectroscopy.
p-nuclei are certain proton-rich, naturally occurring isotopes of some elements between selenium and mercury inclusive which cannot be produced in either the s- or the r-process.

A Borromean nucleus is an atomic nucleus comprising three bound components in which any subsystem of two components is unbound. This has the consequence that if one component is removed, the remaining two comprise an unbound resonance, so that the original nucleus is split into three parts.









