This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these template messages) |

This is a history of the lithium-ion battery.
This article has multiple issues. Please help improve it or discuss these issues on the talk page . (Learn how and when to remove these template messages) |

This is a history of the lithium-ion battery.
Batteries with metallic lithium electrodes presented safety issues, most importantly the formation of lithium dendrites, that internally short-circuit the battery resulting in explosions. Also, dendrites often lose electronic contact with current collectors leading to a loss of cyclable Li+ charge. [11] Consequently, research moved to develop batteries in which, instead of metallic lithium, only lithium compounds are present, being capable of accepting and releasing lithium ions.


In 2017 (2 years before the 2019 Nobel Prize in Chemistry was awarded) George Blomgren offered some speculations on why Akira Yoshino's group produced a commercially viable lithium-ion battery before Jeff Dahn's group: [49]
The performance and capacity of lithium-ion batteries increased as development progressed.

Industry produced about 660 million cylindrical lithium-ion cells in 2012; the 18650 size is by far the most popular for cylindrical cells. If Tesla were to have met its goal of shipping 40,000 Model S electric cars in 2014 and if the 85-kWh battery, which uses 7,104 of these cells, had proved as popular overseas as it was in the United States, a 2014 study projected that the Model S alone would use almost 40 percent of estimated global cylindrical battery production during 2014. [79] As of 2013 [update] , production was gradually shifting to higher-capacity 3,000+ mAh cells. Annual flat polymer cell demand was expected to exceed 700 million in 2013. [80] [ needs update ]
Prices of lithium-ion batteries have fallen over time. Overall, between 1991 and 2018, prices for all types of lithium-ion cells (in dollars per kWh) fell approximately 97%. [77] Over the same time period, energy density more than tripled. [77] Efforts to increase energy density contributed significantly to cost reduction. [81]
In 2015, cost estimates ranged from $300–500/kWh[ clarification needed ]. [82] In 2016 GM revealed they would be paying US$145/kWh for the batteries in the Chevy Bolt EV. [83] In 2017, the average residential energy storage systems installation cost was expected to drop from $1600 /kWh in 2015 to $250 /kWh by 2040 and to see the price with 70% reduction by 2030. [84] In 2019, some electric vehicle battery pack costs were estimated at $150–200, [85] and VW noted it was paying US$100/kWh for its next generation of electric vehicles. [86]
Batteries are used for grid energy storage and ancillary services. For a Li-ion storage coupled with photovoltaics and an anaerobic digestion biogas power plant, Li-ion will generate a higher profit if it is cycled more frequently (hence a higher lifetime electricity output) although the lifetime is reduced due to degradation. [87]
Several types of lithium nickel cobalt manganese oxide (NCM) and lithium nickel cobalt aluminium oxide (NCA) cathode powders with a layered structure are commercially available. Their chemical compositions are specified by the molar ratio of component metals. NCM 111 (or NCM 333) have equimolar parts of nickel, cobalt and manganese. Notably, in NCM cathodes, manganese is not electroactive and remains in the oxidation state +4 during battery's charge-discharge cycling. Cobalt is cycled between the oxidation states +3 and +4, and nickel - between +2 and +4. Due to the higher price of cobalt and due to the higher number of cyclable electrons per nickel atom, high-nickel(also known as "nickel-rich") materials (with Ni atomic percentage > 50%) gain considerable attention from both battery researchers and battery manufacturers. However, high-Ni cathodes are prone to O2 evolution and Li+/Ni4+ cation mixing upon overcharging. [88]
As of 2019 [update] , NMC 532 and NMC 622 were the preferred low-cobalt types for electric vehicles, with NMC 811 and even lower cobalt ratios seeing increasing use, mitigating cobalt dependency. [89] [90] [85] However, cobalt for electric vehicles increased 81% from the first half of 2018 to 7,200 tonnes in the first half of 2019, for a battery capacity of 46.3 GWh. [91]
In 2010, global lithium-ion battery production capacity was 20 gigawatt-hours. [92] By 2016, it was 28 GWh, with 16.4 GWh in China. [93] Production in 2021 is estimated by various sources to be between 200 and 600 GWh, and predictions for 2023 range from 400 to 1,100 GWh. [94]
An antitrust-violating price-fixing cartel among nine corporate families, including LG Chem, GS Yuasa, Hitachi Maxell, NEC, Panasonic/Sanyo, Samsung, Sony, and Toshiba was found to be rigging battery prices and restricting output between 2000 and 2011. [95] [96] [97] [98]

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

Michael Stanley Whittingham is a British-American chemist. He is a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, State University of New York. He also serves as director of the Northeastern Center for Chemical Energy Storage (NECCES) of the U.S. Department of Energy at Binghamton. He was awarded the Nobel Prize in Chemistry in 2019 alongside Akira Yoshino and John B. Goodenough.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.

Lithium iron phosphate or lithium ferro-phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations and more recently large grid-scale energy storage.

Lithium cobalt oxide, sometimes called lithium cobaltate or lithium cobaltite, is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.

A solid-state battery is an electrical battery that uses a solid electrolyte to conduct ion movements between the electrodes, instead of the liquid or polymer gel electrolytes found in conventional batteries. Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
A potassium-ion battery or K-ion battery is a type of battery and analogue to lithium-ion batteries, using potassium ions for charge transfer instead of lithium ions. It was invented by the Iranian/American chemist Ali Eftekhari in 2004.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as its charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. Although, in some cases (such as aqueous Na-ion batteries) they are quite different from Li-ion batteries.

Akira Yoshino is a Japanese chemist. He is a fellow of Asahi Kasei Corporation and a professor at Meijo University in Nagoya. He created the first safe, production-viable lithium-ion battery, which became used widely in cellular phones and notebook computers. Yoshino was awarded the Nobel Prize in Chemistry in 2019 alongside M. Stanley Whittingham and John B. Goodenough.
Aluminium-ion batteries are a class of rechargeable battery in which aluminium ions serve as charge carriers. Aluminium can exchange three electrons per ion. This means that insertion of one Al3+ is equivalent to three Li+ ions. Thus, since the ionic radii of Al3+ (0.54 Å) and Li+ (0.76 Å) are similar, significantly higher numbers of electrons and Al3+ ions can be accepted by cathodes with little damage. Al has 50 times (23.5 megawatt-hours m-3) the energy density of Li and is even higher than coal.
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
Magnesium batteries are batteries that utilize magnesium cations as charge carriers and possibly in the anode in electrochemical cells. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.
The glass battery is a type of solid-state battery. It uses a glass electrolyte and lithium or sodium metal electrodes. The battery was invented by John B. Goodenough, inventor of the lithium cobalt oxide and lithium iron phosphate electrode materials used in the lithium-ion battery (Li-ion), and Maria H. Braga, an associate professor at the University of Porto and a senior research fellow at Cockrell School of Engineering at The University of Texas.
Calcium (ion) batteries are energy storage and delivery technologies (i.e., electro–chemical energy storage) that employ calcium ions (cations), Ca2+, as the active charge carrier. Calcium (ion) batteries remain an active area of research, with studies and work persisting in the discovery and development of electrodes and electrolytes that enable stable, long-term battery operation.
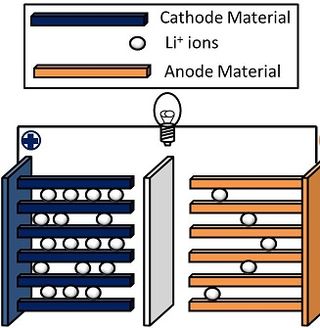
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.
Arumugam Manthiram is an Indian-American materials scientist and engineer, best known for his identification of the polyanion class of lithium ion battery cathodes, understanding of how chemical instability limits the capacity of layered oxide cathodes, and technological advances in lithium sulfur batteries. He is a Cockrell Family Regents Chair in engineering, Director of the Texas Materials Institute, the Director of the Materials Science and Engineering Program at the University of Texas at Austin, and a former lecturer of Madurai Kamaraj University. Manthiram delivered the 2019 Nobel Lecture in Chemistry on behalf of Chemistry Laureate John B. Goodenough.
811 is rapidly gaining ground on two other slightly less low-cobalt variants, NMC 532 and 622
Industry has been improving NMC technology by steadily increasing the nickel content in each cathode generation (e.g. NMC 433, NMC 532, or the most recent NMC 622)
global lithium-ion battery production from about 20GWh (~6.5bn€) in 2010