
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent functional group derived from isoxazole.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
Pentazole is an aromatic molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has the molecular formula HN5. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazole, and pentazole.
Pyrazole is an organic compound of azole group with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.

A persistent carbene is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet, but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC), in which nitrogen atoms flank the formal carbene.
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
In organic chemistry, umpolung or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach and E.J. Corey. Polarity analysis during retrosynthetic analysis tells a chemist when umpolung tactics are required to synthesize a target molecule.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
A spiropyran is a type of organic chemical compound, known for photochromic properties that provide this molecule with the ability of being used in medical and technological areas. Spiropyrans were discovered in the early twentieth century. However, it was in the middle twenties when Fisher and Hirshbergin observed their photochromic characteristics and reversible reaction. In 1952, Fisher and co-workers announced for the first time photochromism in spiropyrans. Since then, there have been many studies on photochromic compounds that have continued up to the present.
The Davis–Beirut reaction is N,N-bond forming heterocyclization that creates numerous types of 2H-indazoles and indazolones in both acidic and basic conditions The Davis–Beirut reaction is named after Mark Kurth and Makhluf Haddadin's respective universities; University of California, Davis and American University of Beirut, and is appealing because it uses inexpensive starting materials and does not require toxic metals.
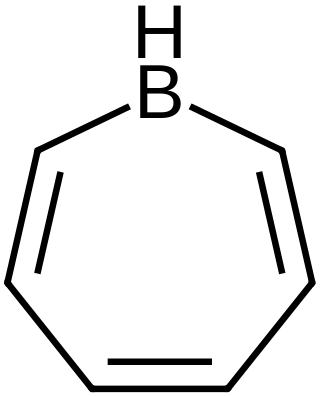
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene. This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid. The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high fluorescence and potential applications in technologies like organic light-emitting diodes (OLEDs) and photovoltaic cells.




![Marsanidine [1034875-38-1] Marsanidine.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/2/25/Marsanidine.svg/220px-Marsanidine.svg.png)







