In chemistry, a disulfide is a compound containing a R−S−S−R′ functional group or the S2−
2 anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".
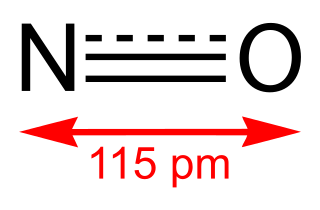
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.

Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. NO is an important cellular signaling molecule. It helps modulate vascular tone, insulin secretion, airway tone, and peristalsis, and is involved in angiogenesis and neural development. It may function as a retrograde neurotransmitter. Nitric oxide is mediated in mammals by the calcium-calmodulin controlled isoenzymes eNOS and nNOS. The inducible isoform, iNOS, involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism, as NO is a free radical with an unpaired electron. It is the proximate cause of septic shock and may function in autoimmune disease.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Nitrosation is a process of converting organic compounds into nitroso derivatives, i.e., compounds containing the R-NO functionality.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.
Gasotransmitters is a class of neurotransmitters. The molecules are distinguished from other bioactive endogenous gaseous signaling molecules based on a need to meet distinct characterization criteria. Currently, only nitric oxide, carbon monoxide, and hydrogen sulfide are accepted as gasotransmitters. According to in vitro models, gasotransmitters, like other gaseous signaling molecules, may bind to gasoreceptors and trigger signaling in the cells.
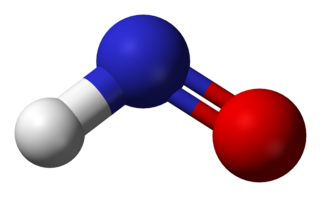
Nitroxyl or azanone is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in the solution phase. The conjugate base, NO−, nitroxide anion, is the reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. The bond dissociation energy of H−NO is 49.5 kcal/mol (207 kJ/mol), which is unusually weak for a bond to the hydrogen atom.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent, after William A. Tilden, who was the first to produce it as a pure compound.

S-Nitroso-N-acetylpenicillamine (SNAP) is the organosulfur compound with the formula ONSC(CH3)2CH(NHAc)CO2H. It is a green solid.

Reactive nitrogen species (RNS) are a family of antimicrobial molecules derived from nitric oxide (•NO) and superoxide (O2•−) produced via the enzymatic activity of inducible nitric oxide synthase 2 (NOS2) and NADPH oxidase respectively. NOS2 is expressed primarily in macrophages after induction by cytokines and microbial products, notably interferon-gamma (IFN-γ) and lipopolysaccharide (LPS).
In enzymology, a formaldehyde dehydrogenase (EC 1.2.1.46) is an enzyme that catalyzes the chemical reaction

Nitrosylation is the general term for covalent incorporation of a nitric oxide "nitrosyl" moiety into another molecule. There are multiple chemical mechanisms by which this can be achieved; including biological enzymes and industrial processes. The biological functions of nitrosylation are particularly important as S-nitrosylation, the conjugation of NO to cysteine thiols in proteins, is an important part of cell signalling. Coordination of NO to transition metals to give metal nitrosyl complexes, is also referred to as nitrosylation.
Biological functions of nitric oxide are roles that nitric oxide plays within biology.
In biochemistry, S-nitrosylation is the covalent attachment of a nitric oxide group to a cysteine thiol within a protein to form an S-nitrosothiol (SNO). S-Nitrosylation has diverse regulatory roles in bacteria, yeast and plants and in all mammalian cells. It thus operates as a fundamental mechanism for cellular signaling across phylogeny and accounts for the large part of NO bioactivity.

S-Nitrosoglutathione (GSNO) is an endogenous S-nitrosothiol (SNO) that plays a critical role in nitric oxide (NO) signaling and is a source of bioavailable NO. NO coexists in cells with SNOs that serve as endogenous NO carriers and donors. SNOs spontaneously release NO at different rates and can be powerful terminators of free radical chain propagation reactions, by reacting directly with ROO• radicals, yielding nitro derivatives as end products. NO is generated intracellularly by the nitric oxide synthase (NOS) family of enzymes: nNOS, eNOS and iNOS while the in vivo source of many of the SNOs is unknown. In oxygenated buffers, however, formation of SNOs is due to oxidation of NO to dinitrogen trioxide (N2O3). Some evidence suggests that both exogenous NO and endogenously derived NO from nitric oxide synthases can react with glutathione to form GSNO.
Gaseous signaling molecules are gaseous molecules that are either synthesized internally (endogenously) in the organism, tissue or cell or are received by the organism, tissue or cell from outside and that are used to transmit chemical signals which induce certain physiological or biochemical changes in the organism, tissue or cell. The term is applied to, for example, oxygen, carbon dioxide, sulfur dioxide, nitrous oxide, hydrogen cyanide, ammonia, methane, hydrogen, ethylene, etc.

Jonathan Solomon Stamler is an English-born American physician and scientist. He is known for his discovery of protein S-nitrosylation, the addition of a nitric oxide (NO) group to cysteine residues in proteins, as a ubiquitous cellular signal to regulate enzymatic activity and other key protein functions in bacteria, plants and animals, and particularly in transporting NO on cysteines in hemoglobin as the third gas in the respiratory cycle.













