Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It is also referred to as banapple gas.

Sodium nitrate is the chemical compound with the formula NaNO
3. This alkali metal nitrate salt is also known as Chile saltpeter to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine, nitratite or soda niter.

Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.

Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or entirely within one organism, as in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.

Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products.
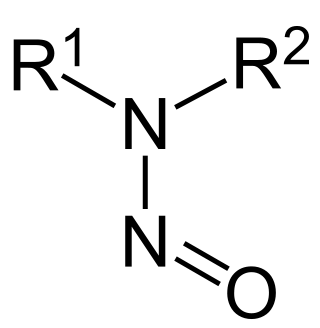
In organic chemistry, nitrosamines are organic compounds with the chemical structure R2N−N=O, where R is usually an alkyl group. They feature a nitroso group bonded to a deprotonated amine. Most nitrosamines are carcinogenic in nonhuman animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive".

Butyl nitrite is the organic compound with the formula CH3(CH2)3ONO. It is an alkyl nitrite made from n-butanol. Butyl nitrite is used recreationally as poppers. Synonyms include 1-butyl nitrite, n-butyl nitrite and nitrous acid butyl ester.
Popper is a slang term given broadly to recreational drug of the chemical class called alkyl nitrites that are inhaled. They act on the body as vasodilators. Most widely sold products include the original isoamyl nitrite, isopentyl nitrite, and isopropyl nitrite. Isobutyl nitrite is also widely used but is banned in the European Union. In some countries, poppers are labeled or packaged as room deodorizers, leather polish, nail polish remover, or videotape head cleaner to evade anti-drug laws.
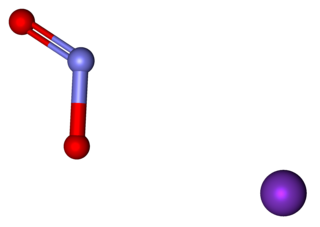
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.
Isobutyl nitrite, C4H9NO2, is an alkyl nitrite, an ester of isobutanol and nitrous acid. Its chemical structure is (CH3)2CH-CH2-ONO.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

Optic pit, optic nerve pit, or optic disc pit (ODP) is rare a congenital excavation (or regional depression) of the optic disc (also optic nerve head), resulting from a malformation during development of the eye. The incidence of ODP is 1 in 10,000 people with no predilection for either gender. There is currently no known risk factors for their development. Optic pits are important because they are associated with posterior vitreous detachments (PVD) and even serous retinal detachments.
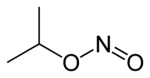
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. They all have the formula C3H8O.

Chloroquine retinopathy is a form of toxic retinopathy caused by the drugs chloroquine or hydroxychloroquine, which are sometimes used in the treatment of autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus. This eye toxicity limits long-term use of the drugs.
Sickle cell retinopathy can be defined as retinal changes due to blood vessel damage in the eye of a person with a background of sickle cell disease. It can likely progress to loss of vision in late stages due to vitreous hemorrhage or retinal detachment. Sickle cell disease is a structural red blood cell disorder leading to consequences in multiple systems. It is characterized by chronic red blood cell destruction, vascular injury, and tissue ischemia causing damage to the brain, eyes, heart, lungs, kidneys, spleen, and musculoskeletal system.
Ocular hypotony, or ocular hypotension, or shortly hypotony, is the medical condition in which intraocular pressure (IOP) of the eye is very low.
Drug abuse retinopathy is damage to the retina of the eyes caused by chronic drug abuse. Types of retinopathy caused by drug abuse include maculopathy, Saturday night retinopathy, and talc retinopathy. Common symptoms include temporary and permanent vision loss, blurred vision, and night blindness. Substances commonly associated with this condition include poppers, heroin, cocaine, methamphetamine, tobacco, and alcohol.
Hexyl nitrite has the formula C6H13NO2 and is a nitrite and more specifically, an alkyl nitrite. It is an ester of hexanol and nitrous acid. It has the structural formula of: CH3(CH2)5ONO The CAS Registry Number is 638-51-7 and the European Community number 680-102-5. It is REACH and TSCA registered. It is also known as nitrous acid, hexyl ester. It is the aliphatic analogue of cyclohexyl nitrite.