In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture.
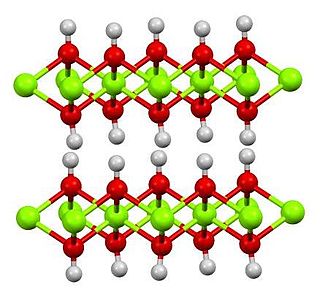
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12). Magnesium hydroxide is a common component of antacids, such as milk of magnesia.

In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Ionic compounds usually form crystalline structures when solid.

Bentonite is an absorbent swelling clay consisting mostly of montmorillonite which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has considerably greater swelling capacity than Ca-montmorillonite.

A solvation shell or solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute. When the solvent is water it is often referred to as a hydration shell or hydration sphere. The number of solvent molecules surrounding each unit of solute is called the hydration number of the solute.

Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina. The particles are plate-shaped with an average diameter around 1 μm and a thickness of 0.96 nm; magnification of about 25,000 times, using an electron microscope, is required to "see" individual clay particles. Members of this group include, amongst others, saponite, nontronite, beidellite, and hectorite.
Electrical mobility is the ability of charged particles to move through a medium in response to an electric field that is pulling them. The separation of ions according to their mobility in gas phase is called ion mobility spectrometry, in liquid phase it is called electrophoresis.
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or angstroms (Å), with 1 Å = 100 pm. Typical values range from 31 pm (0.3 Å) to over 200 pm (2 Å).
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation constant or the solubility of different salts. One of the main characteristics of a solution with dissolved ions is the ionic strength. Ionic strength can be molar or molal and to avoid confusion the units should be stated explicitly. The concept of ionic strength was first introduced by Lewis and Randall in 1921 while describing the activity coefficients of strong electrolytes.
Co-solvents are defined as kosmotropic (order-making) if they contribute to the stability and structure of water-water interactions. In contrast, chaotropic (disorder-making) agents have the opposite effect, disrupting water structure, increasing the solubility of nonpolar solvent particles, and destabilizing solute aggregates. Kosmotropes cause water molecules to favorably interact, which in effect stabilizes intramolecular interactions in macromolecules such as proteins.
Pauling's rules are five rules published by Linus Pauling in 1929 for predicting and rationalizing the crystal structures of ionic compounds.
The lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bind ionic solids. The size of the lattice energy is connected to many other physical properties including solubility, hardness, and volatility. Since it generally cannot be measured directly, the lattice energy is usually deduced from experimental data via the Born–Haber cycle.

The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins.
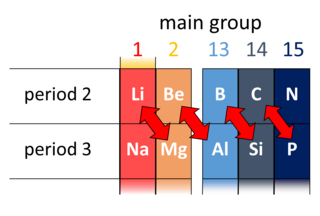
A diagonal relationship is said to exist between certain pairs of diagonally adjacent elements in the second and third periods of the periodic table. These pairs exhibit similar properties; for example, boron and silicon are both semiconductors, forming halides that are hydrolysed in water and have acidic oxides.

Alkali, or Alkaline, soils are clay soils with high pH, a poor soil structure and a low infiltration capacity. Often they have a hard calcareous layer at 0.5 to 1 metre depth. Alkali soils owe their unfavorable physico-chemical properties mainly to the dominating presence of sodium carbonate, which causes the soil to swell and difficult to clarify/settle. They derive their name from the alkali metal group of elements, to which sodium belongs, and which can induce basicity. Sometimes these soils are also referred to as alkaline sodic soils.
Alkaline soils are basic, but not all basic soils are alkaline.

Layered double hydroxides (LDH) are a class of ionic solids characterized by a layered structure with the generic layer sequence [AcB Z AcB]n, where c represents layers of metal cations, A and B are layers of hydroxide anions, and Z are layers of other anions and neutral molecules. Lateral offsets between the layers may result in longer repeating periods.
Resistivity logging is a method of well logging that works by characterizing the rock or sediment in a borehole by measuring its electrical resistivity. Resistivity is a fundamental material property which represents how strongly a material opposes the flow of electric current. In these logs, resistivity is measured using four electrical probes to eliminate the resistance of the contact leads. The log must run in holes containing electrically conductive mud or water, i.e., with enough ions present in the drilling fluid.
In chemistry, ion association is a chemical reaction whereby ions of opposite electrical charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy.
A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in periods 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have a solvation number of 8 or 9. The strength of the bonds between the metal ion and water molecules in the primary solvation shell increases with the electrical charge, z, on the metal ion and decreases as its ionic radius, r, increases. Aqua ions are subject to hydrolysis. The logarithm of the first hydrolysis constant is proportional to z2/r for most aqua ions.










