Related Research Articles

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees.
Sarepta Therapeutics, Inc. is a medical research and drug development company with corporate offices and research facilities in Cambridge, Massachusetts, United States. Incorporated in 1980 as AntiVirals, shortly before going public the company changed its name from AntiVirals to AVI BioPharma soon with stock symbol AVII and in July 2012 changed name from AVI BioPharma to Sarepta Therapeutics and SRPT respectively. As of the end of 2019, the company has two approved drugs.

Peramivir is an antiviral drug developed by BioCryst Pharmaceuticals for the treatment of influenza. Peramivir is a neuraminidase inhibitor, acting as a transition-state analogue inhibitor of influenza neuraminidase and thereby preventing new viruses from emerging from infected cells. It is approved for intravenous administration.

Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza and COVID infections used in Russia and China. The drug is manufactured by Pharmstandard. It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.
BioCryst Pharmaceuticals, Inc. is an American pharmaceutical company headquartered in Durham, North Carolina. The company is a late stage biotech company that focuses on oral drugs for rare and serious diseases. BioCryst's antiviral drug peramivir (Rapivab) was approved by FDA in December 2014. It has also been approved in Japan, Korea, and China.
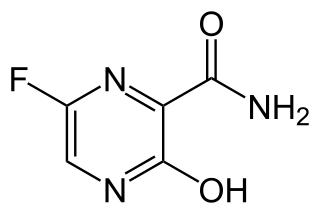
Favipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan. It is also being studied to treat a number of other viral infections, including SARS-CoV-2. Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.

Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.

Galidesivir is an antiviral drug, an adenosine analog. It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.

FGI-106 is a broad-spectrum antiviral drug developed as a potential treatment for enveloped RNA viruses, in particular viral hemorrhagic fevers from the bunyavirus, flavivirus and filovirus families. It acts as an inhibitor which blocks viral entry into host cells. In animal tests FGI-106 shows both prophylactic and curative action against a range of deadly viruses for which few existing treatments are available, including the bunyaviruses hantavirus, Rift Valley fever virus and Crimean-Congo hemorrhagic fever virus, the flavivirus dengue virus, and the filoviruses Ebola virus and Marburg virus.
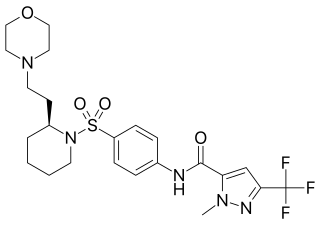
ERDRP-0519 is an antiviral drug which is the first drug specifically developed to target the measles morbillivirus. It acts as an inhibitor of the viral enzyme RNA polymerase which is essential for viral replication, and in animal studies showed good oral bioavailability and protected ferrets from otherwise lethal doses of a morbillivirus when administered up to three days after infection.

The Academy of Military Medical Sciences (AMMS) of the PLA Academy of Military Science is a Chinese military medical research institute. It was established in Shanghai in 1951. It has been based in Beijing since 1958.

There is no cure or specific treatment for the Ebola virus disease that is currently approved for market, although various experimental treatments are being developed. For past and current Ebola epidemics, treatment has been primarily supportive in nature.

Riamilovir is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical. It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.
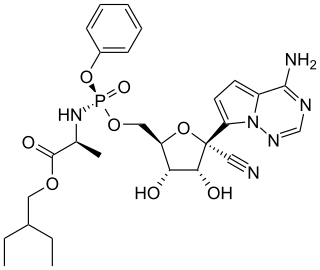
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries.

Baloxavir marboxil, sold under the brand name Xofluza, is an antiviral medication for treatment of influenza A and influenza B flu. It was approved for medical use both in Japan and in the United States in 2018, and is taken as a single dose by mouth. It may reduce the duration of flu symptoms by about a day, but is prone to selection of resistant mutants that render it ineffectual.

Drug repositioning is the repurposing of an approved drug for the treatment of a different disease or medical condition than that for which it was originally developed. This is one line of scientific research which is being pursued to develop safe and effective COVID-19 treatments. Other research directions include the development of a COVID-19 vaccine and convalescent plasma transfusion.

Molnupiravir, sold under the brand name Lagevrio, is an antiviral medication that inhibits the replication of certain RNA viruses. It is used to treat COVID-19 in those infected by SARS-CoV-2. It is taken by mouth.

Coronavir is an anti-viral drug approved in Russia for the treatment of COVID-19. Little is known of this drug, except for the information released by its developer R-Pharm.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.
References
- 1 2 Jourdan A (2014-10-14). "China military-linked firm eyes quick approval of drug to cure Ebola". Reuters.
- ↑ "Suspected Pakuri in "Ebola special drug" developed in Japan WHO points out that the same ingredient is manufactured in China". J-CAST News (in Japanese). Archived from the original on 2014-11-29. Retrieved 2014-11-21.
- ↑ Pinghui Z (1 September 2014). "Ebola virus drug approved on mainland for emergency use only". South China Morning Post.
- ↑ Yang K. "China approves Ebola drug for emergency use". Bioworld.com.
- ↑ "La Chine envoie des médicaments contre Ebola en Afrique". CRI Online (in French). 18 October 2014. Archived from the original on 3 March 2016. Retrieved 19 October 2014.
- ↑ "Sihuan Pharma Company Update" (PDF). 15 October 2014.
- ↑ Sieren F (23 October 2014). "Beijing develops Ebola drug". Deutsche Welle.
- ↑ Wu W, Liu S (October 2014). "[Research progress of prevention and treatment of Ebola virus infection]". Nan Fang Yi Ke da Xue Xue Bao = Journal of Southern Medical University (in Chinese). 34 (10): 1519–22. PMID 25345954.