Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene. [5] [6]
Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene. [5] [6]
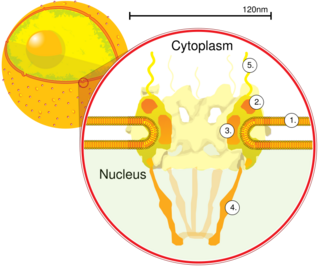
Nucleocytoplasmic transport, a signal- and energy-dependent process, takes place through nuclear pore complexes embedded in the nuclear envelope. The import of proteins containing a classical nuclear localization signal (NLS) requires the NLS import receptor, a heterodimer of importin alpha and beta subunits. Each of these subunits are part of the karyopherin family of proteins. Importin alpha binds the NLS-containing cargo in the cytoplasm and importin beta docks the complex at the cytoplasmic side of the nuclear pore complex. In the presence of nucleoside triphosphates and the small GTP binding protein Ran, the complex moves into the nuclear pore complex and the importin subunits dissociate. Importin alpha enters the nucleoplasm with its passenger protein and importin beta remains at the pore. Interactions between importin beta and the FG repeats of nucleoporins are essential in translocation through the pore complex. The protein encoded by this gene is a member of the importin beta family. [7]
KPNB1 has been shown to interact with:

A nuclear pore is a channel as part of the nuclear pore complex (NPC), a large protein complex found in the nuclear envelope of eukaryotic cells. The nuclear envelope (NE) surrounds the cell nucleus containing DNA and facilitates the selective membrane transport of various molecules.
A nuclear localization signalorsequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus.
Karyopherins are proteins involved in transporting molecules between the cytoplasm and the nucleus of a eukaryotic cell. The inside of the nucleus is called the karyoplasm. Generally, karyopherin-mediated transport occurs through nuclear pores which act as a gateway into and out of the nucleus. Most proteins require karyopherins to traverse the nuclear pore.
Importin is a type of karyopherin that transports protein molecules from the cell's cytoplasm to the nucleus. It does so by binding to specific recognition sequences, called nuclear localization sequences (NLS).

Ran also known as GTP-binding nuclear protein Ran is a protein that in humans is encoded by the RAN gene. Ran is a small 25 kDa protein that is involved in transport into and out of the cell nucleus during interphase and also involved in mitosis. It is a member of the Ras superfamily.
Nuclear transport refers to the mechanisms by which molecules move across the nuclear membrane of a cell. The entry and exit of large molecules from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs). Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with transport factors known as nuclear transport receptors, like karyopherins called importins to enter the nucleus and exportins to exit.
Nuclear pore glycoprotein p62 is a protein complex associated with the nuclear envelope. The p62 protein remains associated with the nuclear pore complex-lamina fraction. p62 is synthesized as a soluble cytoplasmic precursor of 61 kDa followed by modification that involve addition of N-acetylglucosamine residues, followed by association with other complex proteins. In humans it is encoded by the NUP62 gene.

Nucleoporins are a family of proteins which are the constituent building blocks of the nuclear pore complex (NPC). The nuclear pore complex is a massive structure embedded in the nuclear envelope at sites where the inner and outer nuclear membranes fuse, forming a gateway that regulates the flow of macromolecules between the cell nucleus and the cytoplasm. Nuclear pores enable the passive and facilitated transport of molecules across the nuclear envelope. Nucleoporins, a family of around 30 proteins, are the main components of the nuclear pore complex in eukaryotic cells. Nucleoporin 62 is the most abundant member of this family. Nucleoporins are able to transport molecules across the nuclear envelope at a very high rate. A single NPC is able to transport 60,000 protein molecules across the nuclear envelope every minute.

Importin subunit alpha-1 is a protein that in humans is encoded by the KPNA2 gene.
Exportin 1 (XPO1), also known as chromosomal region maintenance 1 (CRM1), is a eukaryotic protein that mediates the nuclear export of various proteins and RNAs.

Importin subunit alpha-5 is a protein that in humans is encoded by the KPNA1 gene.
Nuclear pore complex protein Nup98-Nup96 is a protein that in humans is encoded by the NUP98 gene.

Importin subunit alpha-7 is a protein that in humans is encoded by the KPNA6 gene.
Importin-5 is a protein that in humans is encoded by the IPO5 gene. The protein encoded by this gene is a member of the importin beta family. Structurally, the protein adopts the shape of a right hand solenoid and is composed of 24 HEAT repeats.

RAN binding protein 2 (RANBP2) is protein which in humans is encoded by the RANBP2 gene. It is also known as nucleoporin 358 (Nup358) since it is a member nucleoporin family that makes up the nuclear pore complex. RanBP2 has a mass of 358 kDa.

Nucleoporin 153 (Nup153) is a protein which in humans is encoded by the NUP153 gene. It is an essential component of the basket of nuclear pore complexes (NPCs) in vertebrates, and required for the anchoring of NPCs. It also acts as the docking site of an importing karyopherin. On the cytoplasmic side of the NPC, Nup358 fulfills an analogous role.

Transportin-1 is a protein that in humans is encoded by the TNPO1 gene.

Importin-7 is a protein that in humans is encoded by the IPO7 gene.

Transportin-2 is a protein that in humans is encoded by the TNPO2 gene.
Importin alpha, or karyopherin alpha refers to a class of adaptor proteins that are involved in the import of proteins into the cell nucleus. They are a sub-family of karyopherin proteins.