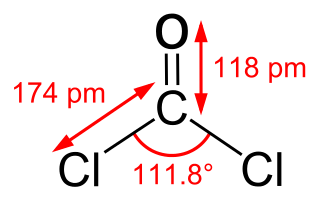
Phosgene is an organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of carbonic acid, or structurally as formaldehyde with the hydrogen atoms replaced by chlorine atoms. Phosgene is a valued and important industrial building block, especially for the production of precursors of polyurethanes and polycarbonate plastics.

Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage.
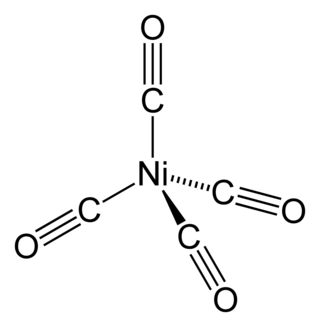
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

White spirit (AU, UK and Ireland) or mineral spirits (US, Canada), also known as mineral turpentine (AU/NZ), turpentine substitute, and petroleum spirits, is a petroleum-derived clear liquid used as a common organic solvent in painting. There are also terms for specific kinds of white spirit, including Stoddard solvent and solvent naphtha (petroleum). White spirit is often used as a paint thinner, or as a component thereof, though paint thinner is a broader category of solvent. Odorless mineral spirits (OMS) have been refined to remove the more toxic aromatic compounds, and are recommended for applications such as oil painting.
The permissible exposure limit is a legal limit in the United States for exposure of an employee to a chemical substance or physical agent such as high level noise. Permissible exposure limits were established by the Occupational Safety and Health Administration (OSHA). Most of OSHA's PELs were issued shortly after adoption of the Occupational Safety and Health (OSH) Act in 1970.
Acute toxicity describes the adverse effects of a substance that result either from a single exposure or from multiple exposures in a short period of time. To be described as acute toxicity, the adverse effects should occur within 14 days of the administration of the substance.

Chemical hazards are typical of hazardous chemicals and hazardous materials in general. Exposure to certain chemicals can cause acute or long-term adverse health effects. Chemical hazards are usually classified separately from biological hazards (biohazards). Main classifications of chemical hazards include asphyxiants, corrosives, irritants, sensitizers, carcinogens, mutagens, teratogens, reactants, and flammables. In the workplace, exposure to chemical hazards is a type of occupational hazard. The use of protective personal equipment (PPE) may substantially reduce the risk of damage from contact with hazardous materials.

Pentaborane(9) is an inorganic compound with the formula B5H9. It is one of the most common boron hydride clusters, although it is a highly reactive compound. Because of its high reactivity with oxygen, it was once evaluated as rocket or jet fuel. Like many of the smaller boron hydrides, pentaborane is colourless, diamagnetic, and volatile. It is related to pentaborane(11).

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.

2-Butoxyethanol is an organic compound with the chemical formula BuOC2H4OH (Bu = CH3CH2CH2CH2). This colorless liquid has a sweet, ether-like odor, as it derives from the family of glycol ethers, and is a butyl ether of ethylene glycol. As a relatively nonvolatile, inexpensive solvent, it is used in many domestic and industrial products because of its properties as a surfactant. It is a known respiratory irritant and can be acutely toxic, but animal studies did not find it to be mutagenic, and no studies suggest it is a human carcinogen. A study of 13 classroom air contaminants conducted in Portugal reported a statistically significant association with increased rates of nasal obstruction and a positive association below the level of statistical significance with a higher risk of obese asthma and increased child BMI.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or "leaking gas", but smells of rotten eggs at higher concentrations.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.

The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is an internationally agreed-upon standard managed by the United Nations that was set up to replace the assortment of hazardous material classification and labelling schemes previously used around the world. Core elements of the GHS include standardized hazard testing criteria, universal warning pictograms, and safety data sheets which provide users of dangerous goods relevant information with consistent organization. The system acts as a complement to the UN numbered system of regulated hazardous material transport. Implementation is managed through the UN Secretariat. Although adoption has taken time, as of 2017, the system has been enacted to significant extents in most major countries of the world. This includes the European Union, which has implemented the United Nations' GHS into EU law as the CLP Regulation, and United States Occupational Safety and Health Administration standards.

Dibutyl phthalate (DBP) is an organic compound which is commonly used as a plasticizer because of its low toxicity and wide liquid range. With the chemical formula C6H4(CO2C4H9)2, it is a colorless oil, although impurities often render commercial samples yellow.
Poisonous material is a material, other than a gas, known to be so toxic to humans that it presents a health hazard during transportation.
Sterilant gas monitoring is the detection of hazardous gases used by health care and other facilities to sterilize medical supplies that cannot be sterilized by heat or steam methods. The current FDA approved sterilant gases are ethylene oxide, hydrogen peroxide and ozone. Other liquid sterilants, such as peracetic acid, may also be used for sterilization and may raise similar occupational health issues. Sterilization means the complete destruction of all biological life, and sterilization efficacy is typically considered adequate if less than one in a million microbes remain viable.

Manure management refers to capture, storage, treatment, and utilization of animal manures in an environmentally sustainable manner. It can be retained in various holding facilities. Animal manure can occur in a liquid, slurry, or solid form. It is utilized by distribution on fields in amounts that enrich soils without causing water pollution or unacceptably high levels of nutrient enrichment. Manure management is a component of nutrient management.
Inhalation is a major route of exposure that occurs when an individual breathes in polluted air which enters the respiratory tract. Identification of the pollutant uptake by the respiratory system can determine how the resulting exposure contributes to the dose. In this way, the mechanism of pollutant uptake by the respiratory system can be used to predict potential health impacts within the human population.
Chlorine gas poisoning is an illness resulting from the effects of exposure to chlorine beyond the threshold limit value.













