
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively.

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, sjögren syndrome and underactive bladder.
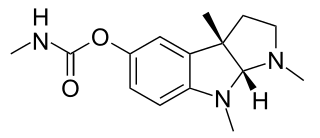
Physostigmine is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and the Manchineel tree.

Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments. It is an alkaloid that has been isolated from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and some other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Methacholine is a synthetic choline ester that acts as a non-selective muscarinic receptor agonist in the parasympathetic nervous system.
Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors.
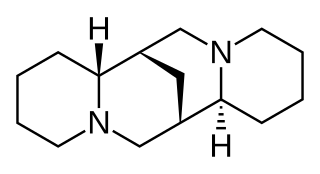
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent cations calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.

Methyllycaconitine (MLA) is a diterpenoid alkaloid found in many species of Delphinium (larkspurs). In common with many other diterpenoid alkaloids, it is toxic to animals, although the acute toxicity varies with species. Early research was focused on identifying, and characterizing the properties of methyllycaconitine as one of the principal toxins in larkspurs responsible for livestock poisoning in the mountain rangelands of North America. Methyllycaconitine has been explored as a possible therapeutic agent for the treatment of spastic paralyses in man, and it has been shown to have insecticidal properties. Most recently, it has become an important molecular probe for studying the pharmacology of the nicotinic acetylcholine receptor.

Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the family Dendrobatidae, notably Oophaga histrionica, which are native to Colombia. It is likely that, as with other poison frog alkaloids, histrionicotoxins are not manufactured by the amphibians, but absorbed from insects in their diet and stored in glands in their skin. They are notably less toxic than other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.
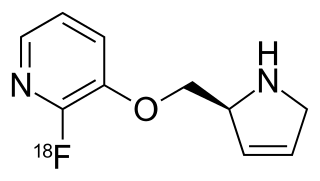
Nifene is a high affinity, selective nicotinic α4β2* receptor partial agonist used in medical research for nicotinic acetylcholine receptors, usually in the form of nifene (18F) as a positron emission tomography (PET) radiotracer.

Methoctramine is a polymethylene tetraamine that acts as a muscarinic antagonist. It preferently binds to the pre-synaptic receptor M2, a muscarinic acetylcholine ganglionic protein complex present basically in heart cells. In normal conditions -absence of methoctramine-, the activation of M2 receptors diminishes the speed of conduction of the sinoatrial and atrioventricular nodes thus reducing the heart rate. Thanks to its apparently high cardioselectivity, it has been studied as a potential parasymphatolitic drug, particularly against bradycardia. However, currently it’s only addressed for research purposes, since the administration to humans is still unavailable.

Anagyrine is a teratogenic alkaloid commonly found in many species of Lupinus plants. The toxin can cause crooked calf disease if a cow ingests the plant during certain periods of pregnancy.
Autonomic drugs can either inhibit or enhance the functions of the parasympathetic and sympathetic nervous systems. This type of drug can be used to treat a wide range of diseases, such as glaucoma, asthma, urinary, gastrointestinal and cardiopulmonary disorders.

Guanitoxin (GNT), formerly known as anatoxin-a(S) "Salivary", is a naturally occurring cyanotoxin commonly isolated from cyanobacteria and causes excess salivation in mammals via inhibition of acetylcholinesterase. Guanitoxin was first structurally characterized in 1989, and consists of a cyclic N-hydroxyguanine organophosphate with a phosphate ester moiety.

Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids.
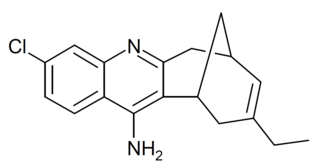
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM, as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors. In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease, and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.























