| MIR34A | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MIR34A , MIRN34A, miRNA34A, mir-34, mir-34a, microRNA 34a, MIRN34 microRNA, human | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 611172 GeneCards: MIR34A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
MicroRNA 34a (miR-34a) is a MicroRNA that in humans is encoded by the MIR34A gene. [3]
| MIR34A | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MIR34A , MIRN34A, miRNA34A, mir-34, mir-34a, microRNA 34a, MIRN34 microRNA, human | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 611172 GeneCards: MIR34A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
MicroRNA 34a (miR-34a) is a MicroRNA that in humans is encoded by the MIR34A gene. [3]
microRNAs (miRNAs) are short (20-24 nt) non-coding RNAs that are involved in post-transcriptional regulation of gene expression in multicellular organisms by affecting both the stability and translation of mRNAs. miRNAs are transcribed by RNA polymerase II as part of capped and polyadenylated primary transcripts (pri-miRNAs) that can be either protein-coding or non-coding.
The primary transcript is cleaved by the Drosha ribonuclease III enzyme to produce an approximately 70-nt stem-loop precursor miRNA (pre-miRNA), which is further cleaved by the cytoplasmic Dicer ribonuclease to generate the mature miRNA and antisense miRNA star (miRNA*) products.
The mature miRNA is incorporated into a RNA-induced silencing complex (RISC), which recognizes target mRNAs through imperfect base pairing with the miRNA and most commonly results in translational inhibition or destabilization of the target mRNA. The RefSeq represents the predicted microRNA stem-loop.
Expression of miR-34A is markedly up-regulated in hematopoietic stem cells (HSCs) from mice subjected to ionizing radiation. [4] HSCs that are deficient in miR-34A have decreased expression of genes involved in the DNA repair processes of homologous recombination and non-homologous end joining. [4] These and other findings demonstrate that miR-34A contributes to the survival of HSCs after irradiation. [4]
miR-34a suppresses the gene expression of the NAMPT gene which encodes the nicotinamide phosphoribosyltransferase (Nampt) enzyme which is the rate-limiting enzyme in the nicotinamide adenine dinucleotide (NAD) salvage pathway, resulting in reduced levels of NAD. [5] miR-34a suppression of NAMPT gene expression also reduces levels of sirtuin 1. [5] Aging and obesity increases levels of miR-34a. [5] The pro-inflammatory transcription factor NF-κB (increasingly expressed with obesity and aging) [6] increases miR-34a expression by binding to its promoter region. [7] Inhibition of miR-34a in diet-induced obese mice restored levels of NAMPT and NAD, reducing inflammation and improving glucose tolerance. [8]
miR-34a represses the translation of sirtuin 1 (SIRT1) in liver by binding to the 3′-UTR region of SIRT1 messenger RNA, contributing to metabolic syndrome [9] [10] Downregulation of SIRT1 by miR-34a promotes cellular senescence and inflammation in vascular smooth muscle cells of old mice, similar to reduced SIRT1 in vascular smooth muscle cells in humans. [11] Impaired endothelial-dependent vasorelaxation caused by miR-34a can be ameliorated by SIRT1 overexpression. [11]

Osteoprotegerin (OPG), also known as osteoclastogenesis inhibitory factor (OCIF) or tumour necrosis factor receptor superfamily member 11B (TNFRSF11B), is a cytokine receptor of the tumour necrosis factor (TNF) receptor superfamily encoded by the TNFRSF11B gene.

Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a family of transcription factor protein complexes that controls transcription of DNA, cytokine production and cell survival. NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. NF-κB has also been implicated in processes of synaptic plasticity and memory.
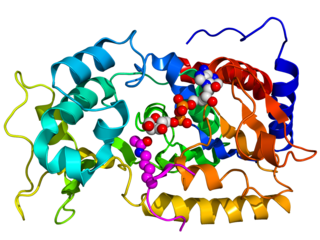
Sirtuins are a family of signaling proteins involved in metabolic regulation. They are ancient in animal evolution and appear to possess a highly conserved structure throughout all kingdoms of life. Chemically, sirtuins are a class of proteins that possess either mono-ADP-ribosyltransferase or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity. The name Sir2 comes from the yeast gene 'silent mating-type information regulation 2', the gene responsible for cellular regulation in yeast.

mir-133 is a type of non-coding RNA called a microRNA that was first experimentally characterised in mice. Homologues have since been discovered in several other species including invertebrates such as the fruitfly Drosophila melanogaster. Each species often encodes multiple microRNAs with identical or similar mature sequence. For example, in the human genome there are three known miR-133 genes: miR-133a-1, miR-133a-2 and miR-133b found on chromosomes 18, 20 and 6 respectively. The mature sequence is excised from the 3' arm of the hairpin. miR-133 is expressed in muscle tissue and appears to repress the expression of non-muscle genes.

There are 89 known sequences today in the microRNA 19 (miR-19) family but it will change quickly. They are found in a large number of vertebrate species. The miR-19 microRNA precursor is a small non-coding RNA molecule that regulates gene expression. Within the human and mouse genome there are three copies of this microRNA that are processed from multiple predicted precursor hairpins:
The miR-34 microRNA precursor family are non-coding RNA molecules that, in mammals, give rise to three major mature miRNAs. The miR-34 family members were discovered computationally and later verified experimentally. The precursor miRNA stem-loop is processed in the cytoplasm of the cell, with the predominant miR-34 mature sequence excised from the 5' arm of the hairpin.

Nicotinamide phosphoribosyltransferase, formerly known as pre-B-cell colony-enhancing factor 1 (PBEF1) or visfatin for its extracellular form (eNAMPT), is an enzyme that in humans is encoded by the NAMPT gene. The intracellular form of this protein (iNAMPT) is the rate-limiting enzyme in the nicotinamide adenine dinucleotide (NAD+) salvage pathway that converts nicotinamide to nicotinamide mononucleotide (NMN) which is responsible for most of the NAD+ formation in mammals. iNAMPT can also catalyze the synthesis of NMN from phosphoribosyl pyrophosphate (PRPP) when ATP is present. eNAMPT has been reported to be a cytokine (PBEF) that activates TLR4, that promotes B cell maturation, and that inhibits neutrophil apoptosis.

Sirtuin 1, also known as NAD-dependent deacetylase sirtuin-1, is a protein that in humans is encoded by the SIRT1 gene.

MiR-155 is a microRNA that in humans is encoded by the MIR155 host gene or MIR155HG. MiR-155 plays a role in various physiological and pathological processes. Exogenous molecular control in vivo of miR-155 expression may inhibit malignant growth, viral infections, and enhance the progression of cardiovascular diseases.
mir-127 microRNA is a short non-coding RNA molecule with interesting overlapping gene structure. miR-127 functions to regulate the expression levels of genes involved in lung development, placental formation and apoptosis. Aberrant expression of miR-127 has been linked to different cancers.
In molecular biology miR-132 microRNA is a short non-coding RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms, generally reducing protein levels through the cleavage of mRNAs or the repression of their translation. Several targets for miR-132 have been described, including mediators of neurological development, synaptic transmission, inflammation and angiogenesis.

miR-33 is a family of microRNA precursors, which are processed by the Dicer enzyme to give mature microRNAs. miR-33 is found in several animal species, including humans. In some species there is a single member of this family which gives the mature product mir-33. In humans there are two members of this family called mir-33a and mir-33b, which are located in intronic regions within two protein-coding genes for Sterol regulatory element-binding proteins respectively.
In recent years it has become apparent that the environment and underlying mechanisms affect gene expression and the genome outside of the central dogma of biology. It has been found that many epigenetic mechanisms are involved in the regulation and expression of genes such as DNA methylation and chromatin remodeling. These epigenetic mechanisms are believed to be a contributing factor to pathological diseases such as type 2 diabetes. An understanding of the epigenome of Diabetes patients may help to elucidate otherwise hidden causes of this disease.
In molecular biology mir-365 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-370 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. This microRNA, mir-370-3p, has been shown to play a role in heart failure. The upregulation of mir-370-3p in the sinus node leads to downregulation of the pacemaker ion channel, HCN4, and thus downregulation of the corresponding ionic current, which causes sinus bradycardia.
In molecular biology mir-23 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
mir-618 microRNA is a short non-coding RNA molecule belonging both to the family of microRNAs and to that of small interfering RNAs (siRNAs). MicroRNAs function to regulate the expression levels of other genes by several mechanisms, whilst siRNAs are involved primarily with the RNA interference (RNAi) pathway.
miR-324-5p is a microRNA that functions in cell growth, apoptosis, cancer, epilepsy, neuronal differentiation, psychiatric conditions, cardiac disease pathology, and more. As a microRNA, it regulates gene expression through targeting mRNAs. Additionally, miR-324-5p is both an intracellular miRNA, meaning it is commonly found within the microenvironment of the cell, and one of several circulating miRNAs found throughout the body. Its presence throughout the body both within and external to cells may contribute to miR-324-5p's wide array of functions and role in numerous disease pathologies – especially cancer – in various organ systems.
Senescence-associated secretory phenotype (SASP) is a phenotype associated with senescent cells wherein those cells secrete high levels of inflammatory cytokines, immune modulators, growth factors, and proteases. SASP may also consist of exosomes and ectosomes containing enzymes, microRNA, DNA fragments, chemokines, and other bioactive factors. Soluble urokinase plasminogen activator surface receptor is part of SASP, and has been used to identify senescent cells for senolytic therapy. Initially, SASP is immunosuppressive and profibrotic, but progresses to become proinflammatory and fibrolytic. SASP is the primary cause of the detrimental effects of senescent cells.
MicroRNA-125 (miR-125) is a highly conserved microRNA family consisting of miR-125a and miR-125b. MiR-125 can be found throughout diverse species from nematode to humans. MiR-125 family members are involved in cell differentiation, proliferation and apoptosis as a result of targeting messenger RNAs related to these cellular processes. By affecting these cellular processes, miR-125 can cause promotion or suppression of pathological processes including carcinogenesis, muscle abnormalities, neurological disorders and pathologies of the immune system. Moreover, miR-125 also plays an important role in normal immune functions and was described to affect development and function of immune cells as well as playing role in immunological host defense in response to bacterial and viral infections.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.