
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.
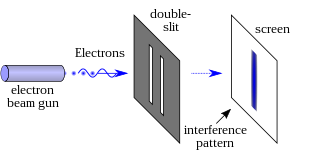
In modern physics, the double-slit experiment demonstrates that light and matter can satisfy the seemingly incongruous classical definitions for both waves and particles. This ambiguity is considered evidence for the fundamentally probabilistic nature of quantum mechanics. This type of experiment was first performed by Thomas Young in 1801, as a demonstration of the wave behavior of visible light. In 1927, Davisson and Germer and, independently George Paget Thomson and his research student Alexander Reid demonstrated that electrons show the same behavior, which was later extended to atoms and molecules. Thomas Young's experiment with light was part of classical physics long before the development of quantum mechanics and the concept of wave–particle duality. He believed it demonstrated that Christiaan Huygens' wave theory of light was correct, and his experiment is sometimes referred to as Young's experiment or Young's slits.
In physics, the fundamental interactions or fundamental forces are the interactions that do not appear to be reducible to more basic interactions. There are four fundamental interactions known to exist:
The holographic principle is a property of string theories and a supposed property of quantum gravity that states that the description of a volume of space can be thought of as encoded on a lower-dimensional boundary to the region — such as a light-like boundary like a gravitational horizon. First proposed by Gerard 't Hooft, it was given a precise string theoretic interpretation by Leonard Susskind, who combined his ideas with previous ones of 't Hooft and Charles Thorn. Leonard Susskind said, "The three-dimensional world of ordinary experience––the universe filled with galaxies, stars, planets, houses, boulders, and people––is a hologram, an image of reality coded on a distant two-dimensional surface." As pointed out by Raphael Bousso, Thorn observed in 1978 that string theory admits a lower-dimensional description in which gravity emerges from it in what would now be called a holographic way. The prime example of holography is the AdS/CFT correspondence.

Quantum mechanics is a fundamental theory in physics that describes the behavior of nature at and below the scale of atoms. It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.

Classical physics is a group of physics theories that predate modern, more complete, or more widely applicable theories. If a currently accepted theory is considered to be modern, and its introduction represented a major paradigm shift, then the previous theories, or new theories based on the older paradigm, will often be referred to as belonging to the area of "classical physics".
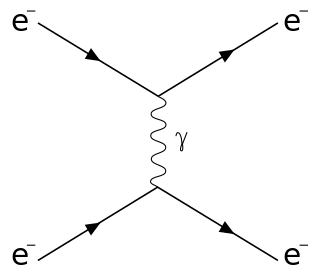
Scattering is a term used in physics to describe a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities in the medium through which they pass. In conventional use, this also includes deviation of reflected radiation from the angle predicted by the law of reflection. Reflections of radiation that undergo scattering are often called diffuse reflections and unscattered reflections are called specular (mirror-like) reflections. Originally, the term was confined to light scattering. As more "ray"-like phenomena were discovered, the idea of scattering was extended to them, so that William Herschel could refer to the scattering of "heat rays" in 1800. John Tyndall, a pioneer in light scattering research, noted the connection between light scattering and acoustic scattering in the 1870s. Near the end of the 19th century, the scattering of cathode rays and X-rays was observed and discussed. With the discovery of subatomic particles and the development of quantum theory in the 20th century, the sense of the term became broader as it was recognized that the same mathematical frameworks used in light scattering could be applied to many other phenomena.
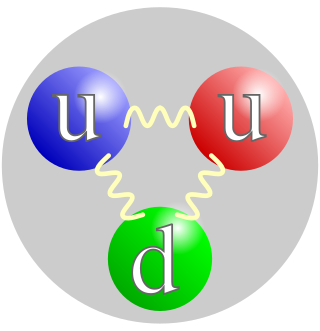
In physics, a subatomic particle is a particle smaller than an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles, or an elementary particle, which is not composed of other particles. Particle physics and nuclear physics study these particles and how they interact. Most force carrying particles like photons or gluons are called bosons and, although they have discrete quanta of energy, do not have rest mass or discrete diameters and are unlike the former particles that have rest mass and cannot overlap or combine which are called fermions.
In physics, quantum tunnelling, barrier penetration, or simply tunnelling is a quantum mechanical phenomenon in which an object such as an electron or atom passes through a potential energy barrier that, according to classical mechanics, should not be passable due to the object not having sufficient energy to pass or surmount the barrier.
In condensed matter physics, a quasiparticle is a concept used to describe a collective behavior of a group of particles that can be treated as if they were a single particle. Formally, quasiparticles and collective excitations are closely related phenomena that arise when a microscopically complicated system such as a solid behaves as if it contained different weakly interacting particles in vacuum.
In quantum mechanics, the measurement problem is the problem of definite outcomes: quantum systems have superpositions but quantum measurements only give one definite result.
In statistical mechanics, the thermodynamic limit or macroscopic limit, of a system is the limit for a large number N of particles where the volume V is taken to grow in proportion with the number of particles. The thermodynamic limit is defined as the limit of a system with a large volume, with the particle density held fixed.
Classical Newtonian physics has, formally, been replaced by quantum mechanics on the small scale and relativity on the large scale. Because most humans continue to think in terms of the kind of events we perceive in the human scale of daily life, it became necessary to provide a new philosophical interpretation of classical physics. Classical mechanics worked extremely well within its domain of observation but made inaccurate predictions at very small scale – atomic scale systems – and when objects moved very fast or were very massive. Viewed through the lens of quantum mechanics or relativity, we can now see that classical physics, imported from the world of our everyday experience, includes notions for which there is no actual evidence. For example, one commonly held idea is that there exists one absolute time shared by all observers. Another is the idea that electrons are discrete entities like miniature planets that circle the nucleus in definite orbits.
Quantum mechanics is the study of matter and its interactions with energy on the scale of atomic and subatomic particles. By contrast, classical physics explains matter and energy only on a scale familiar to human experience, including the behavior of astronomical bodies such as the moon. Classical physics is still used in much of modern science and technology. However, towards the end of the 19th century, scientists discovered phenomena in both the large (macro) and the small (micro) worlds that classical physics could not explain. The desire to resolve inconsistencies between observed phenomena and classical theory led to a revolution in physics, a shift in the original scientific paradigm: the development of quantum mechanics.
The Penrose interpretation is a speculation by Roger Penrose about the relationship between quantum mechanics and general relativity. Penrose proposes that a quantum state remains in superposition until the difference of space-time curvature attains a significant level.

Classical mechanics is a physical theory describing the motion of objects such as projectiles, parts of machinery, spacecraft, planets, stars, and galaxies. The development of classical mechanics involved substantial change in the methods and philosophy of physics. The qualifier classical distinguishes this type of mechanics from physics developed after the revolutions in physics of the early 20th century, all of which revealed limitations in classical mechanics.

In the physical sciences, a particle is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass. They vary greatly in size or quantity, from subatomic particles like the electron, to microscopic particles like atoms and molecules, to macroscopic particles like powders and other granular materials. Particles can also be used to create scientific models of even larger objects depending on their density, such as humans moving in a crowd or celestial bodies in motion.
This glossary of physics is a list of definitions of terms and concepts relevant to physics, its sub-disciplines, and related fields, including mechanics, materials science, nuclear physics, particle physics, and thermodynamics. For more inclusive glossaries concerning related fields of science and technology, see Glossary of chemistry terms, Glossary of astronomy, Glossary of areas of mathematics, and Glossary of engineering.
In 1923, American physicist William Duane presented a discrete momentum-exchange model of the reflection of X-ray photons by a crystal lattice. Duane showed that such a model gives the same scattering angles as the ones calculated via a wave diffraction model, see Bragg's Law.







