Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.

Ammonium fluoride is the inorganic compound with the formula NH4F. It crystallizes as small colourless prisms, having a sharp saline taste, and is highly soluble in water. Like all fluoride salts, it is moderately toxic in both acute and chronic overdose.
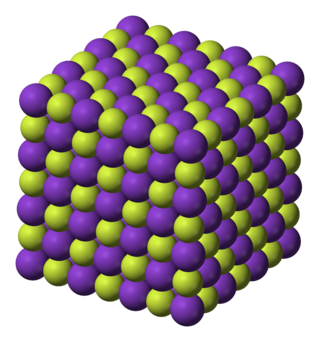
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.

In the area of solid state chemistry. graphite intercalation compounds are materials prepared by intercalation of diverse guests into graphite. The materials have the formula (guest)Cn where n can range from 8 to 40's. The distance between the carbon layers increases significantly upon insertion of the guests. Common guests are reducing agents such as alkali metals. Strong oxidants, such as arsenic pentafluoride also intercalate into graphite. Intercalation involves electron transfer into or out of the host. The properties of these materials differ from those of the parent graphite.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.
The bifluoride ion is an inorganic anion with the chemical formula [HF2]−. The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves electrolysis of bifluoride salts.

Carbon monofluoride (CF, CFx, or (CF)n), also called polycarbon monofluoride (PMF), polycarbon fluoride, poly(carbon monofluoride), and graphite fluoride, is a material formed by high-temperature reaction of fluorine gas with graphite, charcoal, or pyrolytic carbon powder. It is a highly hydrophobic microcrystalline powder. Its CAS number is . In contrast to graphite intercalation compounds it is a covalent graphite compound.

Fluorine is a chemical element; it has symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases.

Potassium bifluoride is the inorganic compound with the formula K[HF2]. This colourless salt consists of the potassium cation and the bifluoride anion. The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products.
Difluorides are chemical compounds with two fluorine atoms per molecule.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
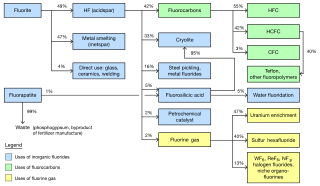
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.
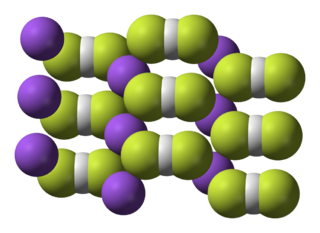
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.

Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3, BrN3, and IN3. The bond between the fluorine atom and the nitrogen is very weak, leading to this substance being very unstable and prone to explosion. Calculations show the F–N–N angle to be around 102° with a straight line of 3 nitrogen atoms.

Astatine compounds are compounds that contain the element astatine (At). As this element is very radioactive, few compounds have been studied. Less reactive than iodine, astatine is the least reactive of the halogens. Its compounds have been synthesized in nano-scale amounts and studied as intensively as possible before their radioactive disintegration. The reactions involved have been typically tested with dilute solutions of astatine mixed with larger amounts of iodine. Acting as a carrier, the iodine ensures there is sufficient material for laboratory techniques to work. Like iodine, astatine has been shown to adopt odd-numbered oxidation states ranging from −1 to +7.
References
- ↑ Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," pp. 25–27.
- ↑ Aigueperse et al. 2005, "Fluorine Compounds, Inorganic," pp. 26–27.
- ↑ Milne, George W. A. (2005). Gardner's commercially important chemicals. John Wiley and Sons. p. 553. ISBN 978-0-471-73518-2.
- ↑ Arora, M. G. (2003). P-block Elements. Anmol Publications. p. 35. ISBN 81-7488-563-3.