Related Research Articles

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.
GB virus C (GBV-C), formerly known as hepatitis G virus (HGV) and also known as human pegivirus – HPgV is a virus in the family Flaviviridae and a member of the Pegivirus, is known to infect humans, but is not known to cause human disease. Reportedly, HIV patients coinfected with GBV-C can survive longer than those without GBV-C, but the patients may be different in other ways. Research is active into the virus' effects on the immune system in patients coinfected with GBV-C and HIV.

DC-SIGN also known as CD209 is a protein which in humans is encoded by the CD209 gene.
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.

The AIDS Clinical Trials Group network (ACTG) is one of the largest HIV clinical trials organizations in the world, playing a major role in setting standards of care for HIV infection and opportunistic diseases related to HIV and AIDS in the United States and the developing world. The ACTG is composed of, and directed by, leading clinical scientists in HIV/AIDS therapeutic research. The ACTG is funded by the Department of Health and Human Services, National Institutes of Health through the National Institute of Allergy and Infectious Diseases.

DNA-directed RNA polymerases I, II, and III subunit RPABC1 is a protein that in humans is encoded by the POLR2E gene.

C-type lectin domain family 4 member M is a protein that in humans is encoded by the CLEC4M gene. CLEC4M has also been designated as CD299.

cAMP-dependent protein kinase catalytic subunit beta is an enzyme that in humans is encoded by the PRKACB gene.

Cyclic AMP-responsive element-binding protein 3 is a protein that in humans is encoded by the CREB3 gene.

Neutral alpha-glucosidase AB is an enzyme that in humans is encoded by the GANAB gene.

DNA dC->dU-editing enzyme APOBEC-3C is a protein that in humans is encoded by the APOBEC3C gene.

Elvucitabine is an experimental nucleoside reverse transcriptase inhibitor (NRTI), developed by Achillion Pharmaceuticals, Inc. for the treatment of HIV infection.

In molecular biology, Tat is a protein that is encoded for by the tat gene in HIV-1. Tat is a regulatory protein that drastically enhances the efficiency of viral transcription. Tat stands for "Trans-Activator of Transcription". The protein consists of between 86 and 101 amino acids depending on the subtype. Tat vastly increases the level of transcription of the HIV dsDNA. Before Tat is present, a small number of RNA transcripts will be made, which allow the Tat protein to be produced. Tat then binds to cellular factors and mediates their phosphorylation, resulting in increased transcription of all HIV genes, providing a positive feedback cycle. This in turn allows HIV to have an explosive response once a threshold amount of Tat is produced, a useful tool for defeating the body's response.

Interferon alpha-16, also known as IFN-alpha-16, is a protein that in humans is encoded by theIFNA16 gene.
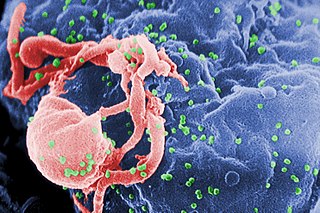
HIV is commonly transmitted via unprotected sexual activity, blood transfusions, hypodermic needles, and from mother to child. Upon acquisition of the virus, the virus replicates inside and kills T helper cells, which are required for almost all adaptive immune responses. There is an initial period of influenza-like illness, and then a latent, asymptomatic phase. When the CD4 lymphocyte count falls below 200 cells/ml of blood, the HIV host has progressed to AIDS, a condition characterized by deficiency in cell-mediated immunity and the resulting increased susceptibility to opportunistic infections and certain forms of cancer.

The stages of HIV infection are acute infection, latency, and AIDS. Acute infection lasts for several weeks and may include symptoms such as fever, swollen lymph nodes, inflammation of the throat, rash, muscle pain, malaise, and mouth and esophageal sores. The latency stage involves few or no symptoms and can last anywhere from two weeks to twenty years or more, depending on the individual. AIDS, the final stage of HIV infection, is defined by low CD4+ T cell counts, various opportunistic infections, cancers, and other conditions.

Stephen C. Harrison is professor of biological chemistry and molecular pharmacology, professor of pediatrics, and director of the Center for Molecular and Cellular Dynamics of Harvard Medical School, head of the Laboratory of Molecular Medicine at Boston Children's Hospital, and investigator of the Howard Hughes Medical Institute.
Topological inhibitors are rigid three-dimensional molecules of inorganic, organic, and hybrid compounds that form multicentered supramolecular interactions in vacant cavities of protein macromolecules and their complexes . Extensive surface and very diverse geometry make cage compounds with an encapsulated metal ion (clathrochelates) suitable for targeting both the active and allosteric sites of enzymes as well as the interfaces of their macromolecular complexes. An efficient structure- and concentration-dependent transcription inhibition in a model in vitro systems based on RNA and DNA polymerases by the iron(II) mono- and bis-clathrochelates at their submicro- and nanomolar concentrations, respectively, is observed in. Molecular docking and preincubation experiments suggested that these cage compounds form supramolecular assemblies with protein residues as well as with DNA and RNA. Thus, they are prospective precursors for the design of antiviral and anticancer drug candidates.
Katherine Seley-Radtke is an American medicinal chemist who specializes in the discovery and design of novel nucleoside or nucleotide based enzyme inhibitors that may be used to treat infections or cancer. She has authored over 90 peer-reviewed publications,is an inventor of five issued US patents, and is a professor in the department of chemistry and biochemistry at the University of Maryland, Baltimore County. Her international impact includes scientific collaborations, policy advising and diplomatic appointments in biosecurity efforts.
References
- 1 2 3 "Division of AIDS Anti-HIV/OI/TB Therapeutics Database". National Institutes of Health, U.S. Department of Health and Human Services. Archived from the original on 2012-01-17. Retrieved 2012-01-24.
- 1 2 Rush M, Huffman D, Noble G, Whiting M, Nasr M (August 25–26, 2011). "2011 Meeting on U.S. Govt. Chem. Databases & Open Chemistry". The NIAID ChemDB HIV/AIDS Database.
- ↑ "Division of AIDS Anti-HIV/OI/TB Therapeutics Database". User Guide. National Institutes of Health, U.S. Department of Health and Human Services. Retrieved 2012-01-24.
- ↑ Hochstein C, Goshorn J, Chang F (2009). "United States National Library of Medicine Drug Information Portal". Med Ref Serv Q. 28 (2): 154–163. doi:10.1080/02763860902816784. PMC 2760770 . PMID 19384716.
- ↑ Cheah EL, Heng PW, Chan LW (2010). "Optimization of supercritical fluid extraction and pressurized liquid extraction of active principles from Magnolis officinalis using the Taguchi design". Sep Purif Technol. 71 (3): 293–301. doi:10.1016/j.seppur.2009.12.009.
- ↑ Portugal J (2009). "Evaluation of molecular descriptors for antitumor drugs with respect to noncovalent binding to DNA and antiproliferative activity". BMC Pharmacol. 9: 11. doi: 10.1186/1471-2210-9-11 . PMC 2758867 . PMID 19758437.
- ↑ Xiong S, Fan J, Kitazato K (2010). "The antiviral protein cyanovirin-N: the current state of its production and applications" (PDF). Appl Microbiol Biotechnol. 86 (3): 805–12. doi:10.1007/s00253-010-2470-1. hdl: 10069/25148 . PMID 20162270. S2CID 35189116.
- ↑ Kremb S, Helfer M, Heller W, Hoffmann D, Wolff H, Kleinschmidt A, Cepok S, Hemmer B, Durner J, Brack-Werner R (2010). "EASY-HIT: HIV full-replication technology for broad discovery of multiple classes of HIV inhibitors". Antimicrob Agents Chemother. 54 (12): 5257–68. doi:10.1128/AAC.00515-10. PMC 2981278 . PMID 20876377.
- ↑ Serafin K, Mazur P, Bak A, Laine E, Tchertanov L, Mouscadet JF, Polanski J (2011). "Ethyl malonate amides: a diketo acid offspring fragment for HIV integrase inhibition". Bioorg Med Chem. 19 (16): 5000–5. doi:10.1016/j.bmc.2011.06.054. PMID 21767953.
- ↑ Durdagi S, Mavromoustakos T, Papdopoulos MG (2008). "3D QSAR CoMFA/CoMSIA, molecular docking and molecular dynamics studies of fullerene-based HIV-1 PR inhibitors". Bioorg Med Chem Lett. 18 (23): 6283–9. doi:10.1016/j.bmcl.2008.09.107. PMID 18951793.
- ↑ Prasanna MD, Vondrasek J, Wlodawer A, Bha TN (2005). "Application of InChI to Curate, Index, and Query 3-D Structure". Proteins. 60 (1): 001–004. doi:10.1002/prot.20469. PMID 15861385. S2CID 14294972.
- ↑ Sánchez R, Morgado E, Grau R (2005). "A genetic code Boolean structure. I. The meaning of Boolean deduction". Bulletin of Mathematical Biology. 67 (1): 001–014. doi:10.1016/j.bulm.2004.05.005. PMID 15691536. S2CID 10480021.
- ↑ Zhang XW (2005). "Generation of predictive pharmacophore model for SARS-coronavirus main proteinase". Eur J Med Chem. 40 (1): 57–62. doi: 10.1016/j.ejmech.2004.09.013 . PMC 7115589 . PMID 15642409.