Related Research Articles
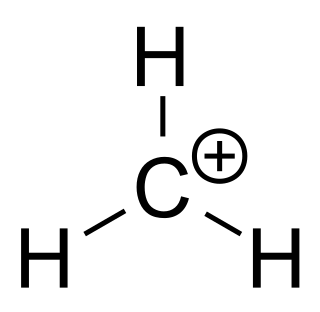
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
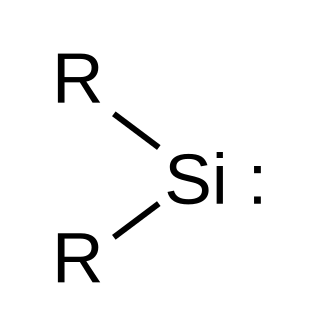
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.
In chemistry, a nitrene or imene is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reactions. The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. The oxidation state of the nitrogen atoms in hydrazoic acid is fractional and is -1/3. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
The von Richter reaction, also named von Richter rearrangement, is a name reaction in the organic chemistry. It is named after Victor von Richter, who discovered this reaction in year 1871. It is the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol to give the product of cine substitution by a carboxyl group. Although it is not generally synthetically useful due to the low chemical yield and formation of numerous side products, its mechanism was of considerable interest, eluding chemists for almost 100 years before the currently accepted one was proposed.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
4-Aminobiphenyl (4-ABP) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.
In organic chemistry, the di-π-methane rearrangement is the photochemical rearrangement of a molecule that contains two π-systems separated by a saturated carbon atom. In the aliphatic case, this molecules is a 1,4-diene; in the aromatic case, an allyl-substituted arene. The reaction forms (respectively) an ene- or aryl-substituted cyclopropane. Formally, it amounts to a 1,2 shift of one ene group or the aryl group, followed by bond formation between the lateral carbons of the non-migrating moiety:
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.

Phosphinidenes are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties. In the last few decades, several strategies have been employed to stabilize phosphinidenes, and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom respectively. They are isoelectronic with the cyclopentadienyl cation C5H+5(Cp+) and comprise four π electrons. Although Hückel's rule cannot be strictly applied to borole, it is considered to be antiaromatic due to having 4 π electrons. As a result, boroles exhibit unique electronic properties not found in other metalloles.
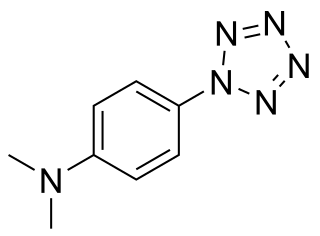
4-Dimethylaminophenylpentazole is an unstable, explosive compound that contains the rare pentazole ring, which is composed of five nitrogen atoms. The electron donating effect of the 4-dimethylamino substituent on the phenyl ring makes this compound one of the more stable of the phenylpentazoles. At room temperature, its chemical half-life is only a few hours, although storage is possible at cryogenic temperatures. The compound was first prepared in 1956 along with other substituted phenylpentazoles. Studies have been conducted on various other derivatives, though necessarily limited by the instability of these compounds. Some more highly substituted derivatives, such as 2,6-dihydroxy-4-dimethylaminophenylpentazole, are slightly more stable but conversely, more difficult to make. Current research has focused on forming transition metal complexes of these pentazole derivatives, as the pentazole ring should be stabilised by bonding to the metal centre.

4-Chlorophenyl azide is an organic aryl azide compound with the chemical formula C6H4ClN3. The geometry between the nitrogen atoms in the azide functional group is approximately linear while the geometry between the nitrogen and the carbon of the benzene is trigonal planar.

9-Fluorenylidene is an aryl carbene derived from the bridging methylene group of fluorene. Fluorenylidene has the unusual property that the triplet ground state is only 1.1 kcal/mol lower in energy than the singlet state. For this reason, fluorenylidene has been studied extensively in organic chemistry.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

Phosphenium ions, not to be confused with phosphonium or phosphirenium, are divalent cations of phosphorus of the form [PR2]+. Phosphenium ions have long been proposed as reaction intermediates.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
References
- ↑ Moss, Robert A.; Platz, Matthew S.; Jones, Maitland Jr, eds. (2004). Reactive Intermediate Chemistry. Wiley. ISBN 9780471233244.[ page needed ]
- ↑ IUPAC , Compendium of Chemical Terminology , 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) " nitrenium ions ". doi : 10.1351/goldbook.N04146
- ↑ de Carvalho, Marcia; Sorrilha, Ana E. P. M.; Rodrigues, J. Augusto R. (1999). "Reaction of aromatic azides with strong acids: Formation of fused nitrogen heterocycles and arylamines" (PDF). Journal of the Brazilian Chemical Society. 10 (5): 415–420. doi: 10.1590/S0103-50531999000500012 .
- ↑ Parks, J. M.; Ford, G. P.; Cramer, C. J. (2001). "Quantum chemical characterization of the reactions of guanine with the phenylnitrenium ion". Journal of Organic Chemistry. 66 (26): 8997–9004. doi:10.1021/jo016066+. PMID 11749633.
- ↑ Borodkin, G I; Shubin, V G (2008-05-31). "Nitrenium ions: structure and reactivity". Russian Chemical Reviews. 77 (5): 395–419. Bibcode:2008RuCRv..77..395B. doi:10.1070/RC2008v077n05ABEH003760. ISSN 0036-021X. S2CID 250845065.
- ↑ Kikugawa, Yasuo (2009). "Application of Stable Nitrenium Ions to Preparative Organic Chemistry". Heterocycles. 78 (3): 571. doi:10.3987/REV-08-644 (inactive 2024-03-07). ISSN 0385-5414.
{{cite journal}}: CS1 maint: DOI inactive as of March 2024 (link) - ↑ Bogdał, Dariusz (2001). "Microwave-assisted generation of carbazolyl nitrenium cation". Arkivoc . 2001 (6): 109–115. doi: 10.3998/ark.5550190.0002.611 . hdl: 2027/spo.5550190.0002.611 .