
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.
The coordination geometry of an atom is the geometrical pattern defined by the atoms around the central atom. The term is commonly applied in the field of inorganic chemistry, where diverse structures are observed. The coodination geometry depends on the number, not the type, of ligands bonded to the metal centre as well as their locations. The number of atoms bonded is the coordination number. The geometrical pattern can be described as a polyhedron where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
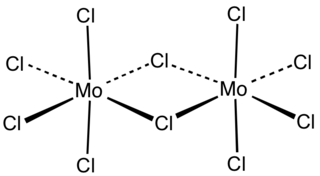
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.
Technetium compounds are chemical compounds containing the chemical element technetium. Technetium can form multiple oxidation states, but often forms in the +4 and +7 oxidation states. Because technetium is radioactive, technetium compounds are extremely rare on Earth.

Tungsten hexacarbonyl (also called tungsten carbonyl) is an organometallic compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.

Molybdenum dichloride describes chemical compounds with the empirical formula MoCl2. At least two forms are known, and both have attracted much attention from academic researchers because of the unexpected structures seen for these compounds and the fact that they give rise to hundreds of derivatives. The form discussed here is Mo6Cl12. The other molybdenum(II) chloride is potassium octachlorodimolybdate.
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
Tungsten(IV) chloride is an inorganic compound with the formula WCl4. It is a diamagnetic black solid. The compound is of interest in research as one of a handful of binary tungsten chlorides.

Tungsten(II) chloride is the inorganic compound with the formula W6Cl12. It is a polymeric cluster compound. The material dissolves in concentrated hydrochloric acid, forming (H3O)2[W6Cl14](H2O)x. Heating this salt gives yellow-brown W6Cl12. The structural chemistry resembles that observed for molybdenum(II) chloride.

Tantalum(III) chloride or tantalum trichloride is non-stoichiometric chemical compound with a range of composition from TaCl2.9 to TaCl3.1 Anionic and neutral clusters containing Ta(III) chloride include [Ta6Cl18]4− and [Ta6Cl14](H2O)4.

Metal cluster compounds are a molecular ion or neutral compound composed of three or more metals and featuring significant metal-metal interactions.
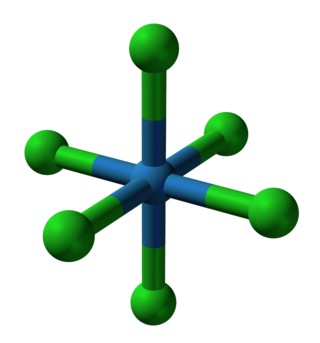
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

In chemistry, a transition metal ether complex is a coordination complex consisting of a transition metal bonded to one or more ether ligand. The inventory of complexes is extensive. Common ether ligands are diethyl ether and tetrahydrofuran. Common chelating ether ligands include the glymes, dimethoxyethane (dme) and diglyme, and the crown ethers. Being lipophilic, metal-ether complexes often exhibit solubility in organic solvents, a property of interest in synthetic chemistry. In contrast, the di-ether 1,4-dioxane is generally a bridging ligand.
![Structure of [M6Cl14] (M = Mo or W). Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png](http://upload.wikimedia.org/wikipedia/commons/thumb/6/69/Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png/220px-Mo6Cl14-anion-from-xtal-2008-CM-3D-ellipsoids.png)
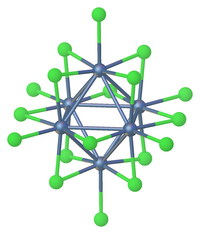
![The complex [Au6C(PPh3)6] , containing a carbon-gold core. Au6C(PPh3)6.png](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5f/Au6C%28PPh3%296.png/240px-Au6C%28PPh3%296.png)














