Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).

Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding of crop strains that were deficient in GA synthesis was one of the key drivers of the "green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is superposable on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.

Succimer, sold under the brand name Chemet among others, is a medication used to treat lead, mercury, and arsenic poisoning. When radiolabeled with technetium-99m, it is used in many types of diagnostic testing. A full course of Succimer lasts for 19 days of oral administration. A second course should be given when more than two weeks pass after the first course.

Zeaxanthin is one of the most common carotenoids in nature, and is used in the xanthophyll cycle. Synthesized in plants and some micro-organisms, it is the pigment that gives paprika, corn, saffron, goji (wolfberries), and many other plants and microbes their characteristic color.
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.

Phenoxy herbicides are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid.

Xylan is a type of hemicellulose, a polysaccharide consisting mainly of xylose residues. It is found in plants, in the secondary cell walls of dicots and all cell walls of grasses. Xylan is the third most abundant biopolymer on Earth, after cellulose and chitin.
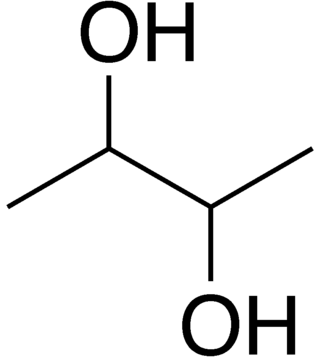
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.

Picenadol (LY-97435) is a 4-phenylpiperidine derivative that is an opioid analgesic drug developed by Eli Lilly in the 1970s.
In enzymology, an ent-copalyl diphosphate synthase is an enzyme that catalyzes the chemical reaction:
The enzyme ent-kaurene synthase catalyzes the chemical reaction
GAI or Gibberellic-Acid Insensitive is a gene in Arabidopsis thaliana which is involved in regulation of plant growth. GAI represses the pathway of gibberellin-sensitive plant growth. It does this by way of its conserved DELLA motif.
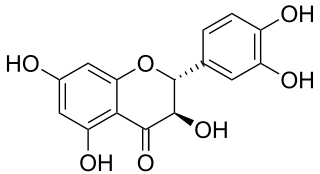
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom. The general formula for an unsubstituted cyclitol is C
nH
2n-x(OH)
x or C
nH
2nO
x where 3 ≤ x ≤ n.
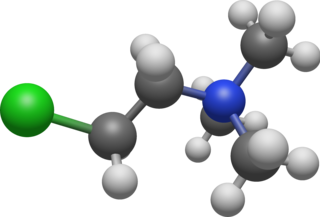
Chlormequat is an organic compound with the formula ClCH
2CH
2N(CH
3)+
3 that is used as a plant growth regulator. It is typically sold as the chloride salt, chlormequat chloride (C5H13Cl2N), a colorless hygroscopic crystalline substance that is soluble in water and ethanol. It is an alkylating agent and a quaternary ammonium salt. Chlormequat is one of the onium-type growth regulators.
Ent-kaurene oxidase (EC 1.14.14.86, Formerly EC 1.14.13.78) is an enzyme with systematic name ent-kaur-16-ene,NADPH:oxygen oxidoreductase (hydroxylating). This enzyme catalyses the following chemical reaction

Fluazifop is the common name used by the ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade.















