
Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:
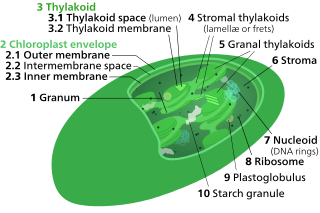
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.

Chloroplasts contain several important membranes, vital for their function. Like mitochondria, chloroplasts have a double-membrane envelope, called the chloroplast envelope, but unlike mitochondria, chloroplasts also have internal membrane structures called thylakoids. Furthermore, one or two additional membranes may enclose chloroplasts in organisms that underwent secondary endosymbiosis, such as the euglenids and chlorarachniophytes.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII.

Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.

Photosystem I is one of two photosystems in the photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein complex that uses light energy to catalyze the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a proton-motive force that is used to generate ATP. PSI is composed of more than 110 cofactors, significantly more than Photosystem II.

In the process of photosynthesis, the phosphorylation of ADP to form ATP using the energy of sunlight is called photophosphorylation. Cyclic photophosphorylation occurs in both aerobic and anaerobic conditions, driven by the main primary source of energy available to living organisms, which is sunlight. All organisms produce a phosphate compound, ATP, which is the universal energy currency of life. In photophosphorylation, light energy is used to pump protons across a biological membrane, mediated by flow of electrons through an electron transport chain. This stores energy in a proton gradient. As the protons flow back through an enzyme called ATP synthase, ATP is generated from ADP and inorganic phosphate. ATP is essential in the Calvin cycle to assist in the synthesis of carbohydrates from carbon dioxide and NADPH.

The cytochrome b6f complex (plastoquinol/plastocyanin reductase or plastoquinol/plastocyanin oxidoreductase; EC 7.1.1.6) is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, that catalyzes the transfer of electrons from plastoquinol to plastocyanin:

Cytochrome f is the largest subunit of cytochrome b6f complex. In its structure and functions, the cytochrome b6f complex bears extensive analogy to the cytochrome bc1 complex of mitochondria and photosynthetic purple bacteria. Cytochrome f plays a role analogous to that of cytochrome c1, in spite of their different structures.

An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts:
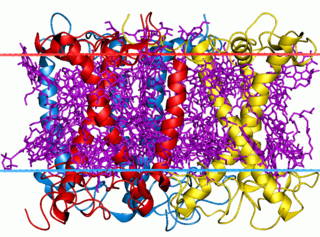
The light-harvesting complex is an array of protein and chlorophyll molecules embedded in the thylakoid membrane of plants and cyanobacteria, which transfer light energy to one chlorophyll a molecule at the reaction center of a photosystem.

A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical energy.

Pheophytin or phaeophytin is a chemical compound that serves as the first electron carrier intermediate in the electron transfer pathway of Photosystem II in plants, and the type II photosynthetic reaction center found in purple bacteria. In both PS II and RC P870, light drives electrons from the reaction center through pheophytin, which then passes the electrons to a quinone (QA) in RC P870 and RC P680. The overall mechanisms, roles, and purposes of the pheophytin molecules in the two transport chains are analogous to each other.
Dioxygen plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis of water during photosynthesis in cyanobacteria, green algae, and plants. During oxidative phosphorylation in cellular respiration, oxygen is reduced to water, thus closing the biological water-oxygen redox cycle.

Light-dependent reactions refers to certain photochemical reactions that are involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions, the first occurs at photosystem II (PSII) and the second occurs at photosystem I (PSI).
SkQ is a class of mitochondria-targeted antioxidants, developed by Professor Vladimir Skulachev and his team. In a broad sense, SkQ is a lipophilic cation, linked via saturated hydrocarbon chain to an antioxidant. Due to its lipophilic properties, SkQ can effectively penetrate through various cell membranes. The positive charge provides directed transport of the whole molecule including antioxidant moiety into the negatively charged mitochondrial matrix. Substances of this type, various drugs that are based on them, as well as methods of their use are patented in Russia and other countries such as United States, China, Japan, and in Europe. Sometimes the term SkQ is used in a narrow sense for the denomination of a cationic derivative of the plant antioxidant plastoquinone.

Chlororespiration is a respiratory process that takes place within plants. Inside plant cells there is an organelle called the chloroplast which is surrounded by the thylakoid membrane. This membrane contains an enzyme called NAD(P)H dehydrogenase which transfers electrons in a linear chain to oxygen molecules. This electron transport chain (ETC) within the chloroplast also interacts with those in the mitochondria where respiration takes place. Photosynthesis is also a process that Chlororespiration interacts with. If photosynthesis is inhibited by environmental stressors like water deficit, increased heat, and/or increased/decreased light exposure, or even chilling stress then chlororespiration is one of the crucial ways that plants use to compensate for chemical energy synthesis.
Plastid terminal oxidase or plastoquinol terminal oxidase (PTOX) is an enzyme that resides on the thylakoid membranes of plant and algae chloroplasts and on the membranes of cyanobacteria. The enzyme was hypothesized to exist as a photosynthetic oxidase in 1982 and was verified by sequence similarity to the mitochondrial alternative oxidase (AOX). The two oxidases evolved from a common ancestral protein in prokaryotes, and they are so functionally and structurally similar that a thylakoid-localized AOX can restore the function of a PTOX knockout.














