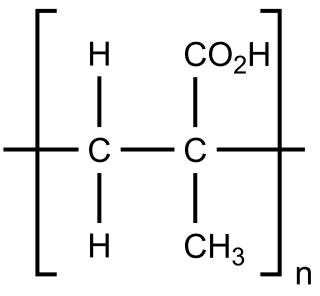
Aspartic acid, is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/neuromodulator.

Ethylenediaminetetraacetic acid (EDTA), also called edetic acid after its own abbreviation, is an aminopolycarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes even at neutral pH. It is thus used to dissolve Fe- and Ca-containing scale as well as to deliver iron ions under conditions where its oxides are insoluble. EDTA is available as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA, but these all function similarly.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.

A hydrogel is a biphasic material, a mixture of porous, permeable solids and at least 10% by weight or volume of interstitial fluid composed completely or mainly by water. In hydrogels the porous permeable solid is a water insoluble three dimensional network of natural or synthetic polymers and a fluid, having absorbed a large amount of water or biological fluids. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894.

Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.

Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. With metals such as sodium and calcium, its salts are known as alginates. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms.
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.
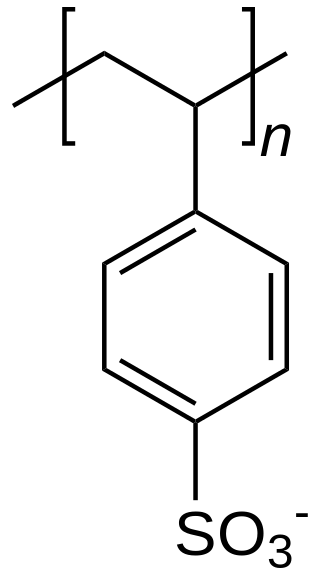
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove potassium, calcium, and sodium from solutions in technical applications.

Tricalcium phosphate (sometimes abbreviated TCP), more commonly known as Calcium phosphate, is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration. It has been used as an additive for food products including bread, juice, and ice cream.

Polyphosphazenes include a wide range of hybrid inorganic-organic polymers with a number of different skeletal architectures with the backbone P-N-P-N-P-N-. In nearly all of these materials two organic side groups are attached to each phosphorus center. Linear polymers have the formula (N=PR1R2)n, where R1 and R2 are organic (see graphic). Other architectures are cyclolinear and cyclomatrix polymers in which small phosphazene rings are connected together by organic chain units. Other architectures are available, such as block copolymer, star, dendritic, or comb-type structures. More than 700 different polyphosphazenes are known, with different side groups (R) and different molecular architectures. Many of these polymers were first synthesized and studied in the research group of Harry R. Allcock.

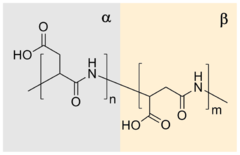
Isoaspartic acid is an aspartic acid residue isomeric to the typical α peptide linkage. It is a β-amino acid, with the side chain carboxyl moved to the backbone. Such a change is caused by a chemical reaction in which the nitrogen atom on the N+1 following peptide bond nucleophilically attacks the γ-carbon of the side chain of an asparagine or aspartic acid residue, forming a succinimide intermediate. Hydrolysis of the intermediate results in two products, either aspartic acid or isoaspartic acid, which is a β-amino acid. The reaction also results in the deamidation of the asparagine residue. Racemization may occur leading to the formation of D-aminoacids.
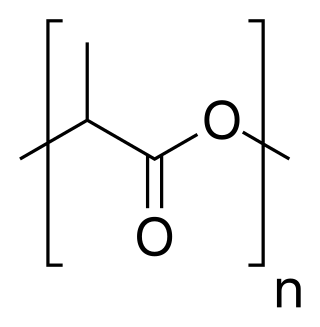
Poly(methacrylic acid) (PMAA) is a polymer made from methacrylic acid (preferred IUPAC name, 2-methylprop-2-enoic acid), which is a carboxylic acid. It is often available as its sodium salt, poly(methacrylic acid) sodium salt. The monomer is a viscous liquid with a pungent odour. The first polymeric form of methacrylic acid was described in 1880 by Engelhorn and Fittig. The use of high purity monomers is required for proper polymerization conditions and therefore it is necessary to remove any inhibitors by extraction (phenolic inhibitors) or via distillation. To prevent inhibition by dissolved oxygen, monomers should be carefully degassed prior to the start of the polymerization.

Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

Edible packaging refers to packaging which is edible and biodegradable.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.
Common hydrogel agriculture's ingredient is potassium polyacrylate or sodium polyacrylate. As a superabsorbent material, it can absorb plenty of water and turn water to gel to store water.
Polysuccinimide (PSI), also known as polyanhydroaspartic acid or polyaspartimide, is formed during the thermal polycondensation of aspartic acid and is the simplest polyimide. Polysuccinimide is insoluble in water, but soluble in some aprotic dipolar solvents. Its reactive nature makes polysuccinimide a versatile starting material for functional polymers made from renewable resources.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.