Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.
Cuprates are a class of compounds that contain copper (Cu) atom(s) in an anion. They can be broadly categorized into two main types:

Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.

The cyanate ion is an anion with the chemical formula OCN−. It is a resonance of three forms: [O−−C≡N] (61%) ↔ [O=C=N−] (30%) ↔ [O+≡C−N2−] (4%).

Sodium molybdate, Na2MoO4, is useful as a source of molybdenum. This white, crystalline salt is often encountered as the dihydrate, Na2MoO4·2H2O.

A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types of covalent bonds: double bonds and triple bonds. Stable quadruple bonds are most common among the transition metals in the middle of the d-block, such as rhenium, tungsten, technetium, molybdenum and chromium. Typically the ligands that support quadruple bonds are π-donors, not π-acceptors. Quadruple bonds are rare as compared to double bonds and triple bonds, but hundreds of compounds with such bonds have been prepared.

Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s. Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity. Its 26 electrons are arranged in the configuration [Ar]3d64s2, of which the 3d and 4s electrons are relatively close in energy, and thus it can lose a variable number of electrons and there is no clear point where further ionization becomes unprofitable.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Sodium ferrocyanide is the sodium salt of the coordination compound of formula [Fe(CN)6]4−. In its hydrous form, Na4Fe(CN)6 · 10 H2O (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. The yellow color is the color of ferrocyanide anion. Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity (acceptable daily intake 0–0.025 mg/kg body weight). The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide. However, like all ferrocyanide salt solutions, addition of an acid or exposure to UV light can result in the production of hydrogen cyanide gas, which is extremely toxic.

Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.

Potassium octachlorodimolybdate is an inorganic compound with the chemical formula K4[Mo2Cl8]. It is known as a red-coloured, microcrystalline solid. The anion is of historic interest as one of the earliest illustrations of a quadruple bonding. The salt is usually obtained as the pink-coloured dihydrate.
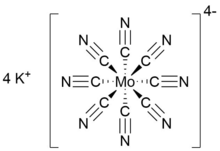
Cyanometallates or cyanometalates are a class of coordination compounds, most often consisting only of cyanide ligands. Most are anions. Cyanide is a highly basic and small ligand, hence it readily saturates the coordination sphere of metal ions. The resulting cyanometallate anions are often used as building blocks for more complex structures called coordination polymers, the best known example of which is Prussian blue, a common dyestuff.
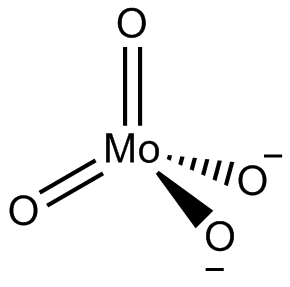
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.

Transition metal thiolate complexes are metal complexes containing thiolate ligands. Thiolates are ligands that can be classified as soft Lewis bases. Therefore, thiolate ligands coordinate most strongly to metals that behave as soft Lewis acids as opposed to those that behave as hard Lewis acids. Most complexes contain other ligands in addition to thiolate, but many homoleptic complexes are known with only thiolate ligands. The amino acid cysteine has a thiol functional group, consequently many cofactors in proteins and enzymes feature cysteinate-metal cofactors.

The cyanonickelates are a class of chemical compound containing anions consisting of nickel atoms, and cyanide groups. The most important of these are the tetracyanonickelates containing four cyanide groups per nickel. The tetracyanonickelates contain the [Ni(CN)4]2− anion. This can exist in solution or in solid salts. The ion has cyanide groups arranged in a square around the central nickel ion. The symmetry of the ion is D4h. The distance from the nickel atom to the carbon is 1.87 Å, and the carbon-nitrogen distance is 1.16 Å. In their crystals, the tetracyanonickelate(II) anions are often arranged in a columnar structure (e.g. in K2[Ni(CN)4]). Tetracyanonickelate(II) can be oxidised electrochemically in solution to yield tetracyanonickelate(III) [Ni(CN)4]−. [Ni(CN)4]− is unstable and Ni(III) oxidises the cyanide to cyanate OCN−. Tetracyanonickelate(III) can add two more cyanide groups to form hexacyanonickelate(III).

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Potassium tetracyanonickelate (IUPAC: Potassium tetracyanido nickelate(II)) is the inorganic compound with the formula K2Ni(CN)4. It is usually encountered as the monohydrate but the anhydrous salt is also known. Both are yellow, water-soluble, diamagnetic solids. The salt consists of potassium ions and the tetracyanonickelate coordination complex, which is square planar. The [Ni(CN)4]2- anions are arranged in a columnar structure with Ni---Ni distances of 4.294 Å, which is well beyond the sum of the van der Waals radius of the nickel cation. This columnar structure resembles those of the other [M(CN)4]2- anions of the heavy congeners of the group 10 metals (M = Pd, Pt).

Potassium diplatinum(II) tetrakispyrophosphite (abbreviated as [Pt2(pop)4]4−) is the inorganic compound with the formula K4[Pt2(HO2POPO2H)4]. It is a water-soluble yellow salt. The compound has a long-lived, strongly luminescent excited state, with an emission maximum at ~510 nm and a lifetime near 10 μs.