
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rocks.

Limonite is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as FeO(OH)·nH2O, although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the three principal iron ores, the others being hematite and magnetite, and has been mined for the production of iron since at least 400 BC.

Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the form of magnetite (Fe
3O
4, 72.4% Fe), hematite (Fe
2O
3, 69.9% Fe), goethite (FeO(OH), 62.9% Fe), limonite (FeO(OH)·n(H2O), 55% Fe) or siderite (FeCO3, 48.2% Fe).

Skarns or tactites are coarse-grained metamorphic rocks that form by replacement of carbonate-bearing rocks during regional or contact metamorphism and metasomatism. Skarns may form by metamorphic recrystallization of impure carbonate protoliths, bimetasomatic reaction of different lithologies, and infiltration metasomatism by magmatic-hydrothermal fluids. Skarns tend to be rich in calcium-magnesium-iron-manganese-aluminium silicate minerals, which are also referred to as calc-silicate minerals. These minerals form as a result of alteration which occurs when hydrothermal fluids interact with a protolith of either igneous or sedimentary origin. In many cases, skarns are associated with the intrusion of a granitic pluton found in and around faults or shear zones that commonly intrude into a carbonate layer composed of either dolomite or limestone. Skarns can form by regional or contact metamorphism and therefore form in relatively high temperature environments. The hydrothermal fluids associated with the metasomatic processes can originate from a variety of sources; magmatic, metamorphic, meteoric, marine, or even a mix of these. The resulting skarn may consist of a variety of different minerals which are highly dependent on both the original composition of the hydrothermal fluid and the original composition of the protolith.

Metasomatism is the chemical alteration of a rock by hydrothermal and other fluids. It is traditionally defined as metamorphism which involves a change in the chemical composition, excluding volatile components. It is the replacement of one rock by another of different mineralogical and chemical composition. The minerals which compose the rocks are dissolved and new mineral formations are deposited in their place. Dissolution and deposition occur simultaneously and the rock remains solid.

Ultramafic rocks are igneous and meta-igneous rocks with a very low silica content, generally >18% MgO, high FeO, low potassium, and are composed of usually greater than 90% mafic minerals. The Earth's mantle is composed of ultramafic rocks. Ultrabasic is a more inclusive term that includes igneous rocks with low silica content that may not be extremely enriched in Fe and Mg, such as carbonatites and ultrapotassic igneous rocks.

Lamprophyres are uncommon, small-volume ultrapotassic igneous rocks primarily occurring as dikes, lopoliths, laccoliths, stocks, and small intrusions. They are alkaline silica-undersaturated mafic or ultramafic rocks with high magnesium oxide, >3% potassium oxide, high sodium oxide, and high nickel and chromium.

Carbonatite is a type of intrusive or extrusive igneous rock defined by mineralogic composition consisting of greater than 50% carbonate minerals. Carbonatites may be confused with marble and may require geochemical verification.

Mineral processing is the process of separating commercially valuable minerals from their ores in the field of extractive metallurgy. Depending on the processes used in each instance, it is often referred to as ore dressing or ore milling.

Various theories of ore genesis explain how the various types of mineral deposits form within Earth's crust. Ore-genesis theories vary depending on the mineral or commodity examined.
In geology, igneous differentiation, or magmatic differentiation, is an umbrella term for the various processes by which magmas undergo bulk chemical change during the partial melting process, cooling, emplacement, or eruption. The sequence of magmas produced by igneous differentiation is known as a magma series.

NASA's 2003 Mars Exploration Rover Mission has amassed an enormous amount of scientific information related to the Martian geology and atmosphere, as well as providing some astronomical observations from Mars. This article covers information gathered by the Opportunity rover during the initial phase of its mission. Information on science gathered by Spirit can be found mostly in the Spirit rover article.
Kambalda type komatiitic nickel ore deposits are a class of magmatic iron-nickel-copper-platinum-group element ore deposit in which the physical processes of komatiite volcanology serve to deposit, concentrate and enrich a Fe-Ni-Cu-(PGE) sulfide melt within the lava flow environment of an erupting komatiite volcano.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.
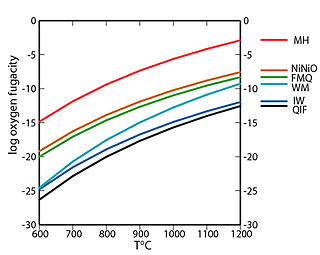
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.
This glossary of geology is a list of definitions of terms and concepts relevant to geology, its sub-disciplines, and related fields. For other terms related to the Earth sciences, see Glossary of geography terms.
Magmatic water, also known as juvenile water, is an aqueous phase in equilibrium with minerals that have been dissolved by magma deep within the Earth's crust and is released to the atmosphere during a volcanic eruption. It plays a key role in assessing the crystallization of igneous rocks, particularly silicates, as well as the rheology and evolution of magma chambers. Magma is composed of minerals, crystals and volatiles in varying relative natural abundance. Magmatic differentiation varies significantly based on various factors, most notably the presence of water. An abundance of volatiles within magma chambers decreases viscosity and leads to the formation of minerals bearing halogens, including chloride and hydroxide groups. In addition, the relative abundance of volatiles varies within basaltic, andesitic, and rhyolitic magma chambers, leading to some volcanoes being exceedingly more explosive than others. Magmatic water is practically insoluble in silicate melts but has demonstrated the highest solubility within rhyolitic melts. An abundance of magmatic water has been shown to lead to high-grade deformation, altering the amount of δ18O and δ2H within host rocks.

Iron-rich sedimentary rocks are sedimentary rocks which contain 15 wt.% or more iron. However, most sedimentary rocks contain iron in varying degrees. The majority of these rocks were deposited during specific geologic time periods: The Precambrian, the early Paleozoic, and the middle to late Mesozoic. Overall, they make up a very small portion of the total sedimentary record.
Mount Ngualla, often referred to simply as Ngualla, is a collapsed volcano located in the remote south west of Tanzania. It is approximately 200 km north of the Mbeya township.















