
The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.

In neuroanatomy, the optic chiasm, or optic chiasma, is the part of the brain where the optic nerves cross. It is located at the bottom of the brain immediately inferior to the hypothalamus. The optic chiasm is found in all vertebrates, although in cyclostomes, it is located within the brain.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

In neuroanatomy, the lateral geniculate nucleus is a structure in the thalamus and a key component of the mammalian visual pathway. It is a small, ovoid, ventral projection of the thalamus where the thalamus connects with the optic nerve. There are two LGNs, one on the left and another on the right side of the thalamus. In humans, both LGNs have six layers of neurons alternating with optic fibers.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

As a part of the retina, bipolar cells exist between photoreceptors and ganglion cells. They act, directly or indirectly, to transmit signals from the photoreceptors to the ganglion cells.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.
Axon guidance is a subfield of neural development concerning the process by which neurons send out axons to reach their correct targets. Axons often follow very precise paths in the nervous system, and how they manage to find their way so accurately is an area of ongoing research.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of ipRGCs was first suspected in 1927 when rodless, coneless mice still responded to a light stimulus through pupil constriction, This implied that rods and cones are not the only light-sensitive neurons in the retina. Yet research on these cells did not advance until the 1980s. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore, they constitute a third class of photoreceptors, in addition to rod and cone cells.
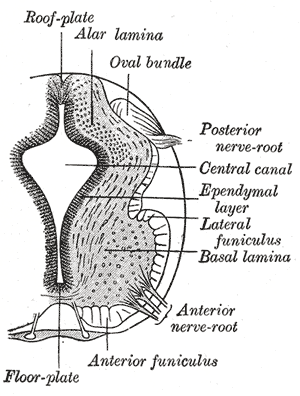
The floor plate is a structure integral to the developing nervous system of vertebrate organisms. Located on the ventral midline of the embryonic neural tube, the floor plate is a specialized glial structure that spans the anteroposterior axis from the midbrain to the tail regions. It has been shown that the floor plate is conserved among vertebrates, such as zebrafish and mice, with homologous structures in invertebrates such as the fruit fly Drosophila and the nematode C. elegans. Functionally, the structure serves as an organizer to ventralize tissues in the embryo as well as to guide neuronal positioning and differentiation along the dorsoventral axis of the neural tube.
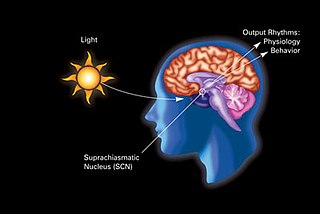
In neuroanatomy, the retinohypothalamic tract (RHT) is a photic neural input pathway involved in the circadian rhythms of mammals. The origin of the retinohypothalamic tract is the intrinsically photosensitive retinal ganglion cells (ipRGC), which contain the photopigment melanopsin. The axons of the ipRGCs belonging to the retinohypothalamic tract project directly, monosynaptically, to the suprachiasmatic nuclei (SCN) via the optic nerve and the optic chiasm. The suprachiasmatic nuclei receive and interpret information on environmental light, dark and day length, important in the entrainment of the "body clock". They can coordinate peripheral "clocks" and direct the pineal gland to secrete the hormone melatonin.

Ephrins are a family of proteins that serve as the ligands of the Eph receptor. Eph receptors in turn compose the largest known subfamily of receptor protein-tyrosine kinases (RTKs).
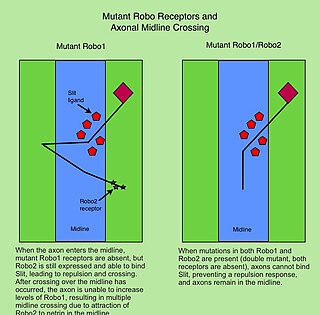
The Roundabout (Robo) family of proteins are single-pass transmembrane receptors that are highly conserved across many branches of the animal kingdom, from C. elegans to humans. They were first discovered in Drosophila, through a mutant screen for genes involved in axon guidance. The Drosophila roundabout mutant was named after its phenotype, which resembled the circular traffic junctions. The Robo receptors are most well known for their role in the development of the nervous system, where they have been shown to respond to secreted Slit ligands. One well-studied example is the requirement for Slit-Robo signaling in regulation of axonal midline crossing. Slit-Robo signaling is also critical for many neurodevelopmental processes including formation of the olfactory tract, the optic nerve, and motor axon fasciculation. In addition, Slit-Robo signaling contributes to cell migration and the development of other tissues such as the lung, kidney, liver, muscle and breast. Mutations in Robo genes have been linked to multiple neurodevelopmental disorders in humans.
Slit is a family of secreted extracellular matrix proteins which play an important signalling role in the neural development of most bilaterians. While lower animal species, including insects and nematode worms, possess a single Slit gene, humans, mice and other vertebrates possess three Slit homologs: Slit1, Slit2 and Slit3. Human Slits have been shown to be involved in certain pathological conditions, such as cancer and inflammation.

A midget cell is one type of retinal ganglion cell (RGC). Midget cells originate in the ganglion cell layer of the retina, and project to the parvocellular layers of the lateral geniculate nucleus (LGN). The axons of midget cells travel through the optic nerve and optic tract, ultimately synapsing with parvocellular cells in the LGN. These cells are known as midget retinal ganglion cells due to the small sizes of their dendritic trees and cell bodies. About 80% of RGCs are midget cells. They receive inputs from relatively few rods and cones. In many cases, they are connected to midget bipolar cells, which are linked to one cone each.
Slit-Robo is the name of a cell signaling protein complex with many diverse functions including axon guidance and angiogenesis.

Ephrin A5 is a protein that in humans is encoded by the EFNA5 gene.

The growth cone is a highly dynamic structure of the developing neuron, changing directionality in response to different secreted and contact-dependent guidance cues; it navigates through the developing nervous system in search of its target. The migration of the growth cone is mediated through the interaction of numerous trophic and tropic factors; netrins, slits, ephrins and semaphorins are four well-studied tropic cues (Fig.1). The growth cone is capable of modifying its sensitivity to these guidance molecules as it migrates to its target; this sensitivity regulation is an important theme seen throughout development.

Retinal precursor cells are biological cells that differentiate into the various cell types of the retina during development. In the vertebrate, these retinal cells differentiate into seven cell types, including retinal ganglion cells, amacrine cells, bipolar cells, horizontal cells, rod photoreceptors, cone photoreceptors, and Müller glia cells. During embryogenesis, retinal cells originate from the anterior portion of the neural plate termed the eye field. Eye field cells with a retinal fate express several transcription factor markers including Rx1, Pax6, and Lhx2. The eye field gives rise to the optic vesicle and then to the optic cup. The retina is generated from the precursor cells within the inner layer of the optic cup, as opposed to the retinal pigment epithelium that originate from the outer layer of the optic cup. In general, the developing retina is organized so that the least-committed precursor cells are located in the periphery of the retina, while the committed cells are located in the center of the retina. The differentiation of retinal precursor cells into the mature cell types found in the retina is coordinated in time and space by factors within the cell as well as factors in the environment of the cell. One example of an intrinsic regulator of this process is the transcription factor Ath5. Ath5 expression in retinal progenitor cells biases their differentiation into a retinal ganglion cell fate. An example of an environmental factor is the morphogen sonic hedge hog (Shh). Shh has been shown to repress the differentiation of precursor cells into retinal ganglion cells.

Forkhead box D1 is a protein that in humans is encoded by the FOXD1 gene. Forkhead d1 is a kidney expressed transcription factor maps at the chromosome 5 at position 5q12—q13, identified in Drosophila forkhead protein and mammalian HNF3 transcription factor. The name of was derived from two spiked head structures in the embryos of Drosophila forkhead mutant. It belong to transcription factor family that displays remarkable functional diversity and involved in a wide variety of biological processes. The most commonly used synonyms for Forkhead D1 are, FOX D1, FREAC-4 and BF2.
















