
The subphylum Chelicerata constitutes one of the major subdivisions of the phylum Arthropoda. Chelicerates include the sea spiders, horseshoe crabs, and arachnids, as well as a number of extinct lineages, such as the eurypterids and chasmataspidids.

Arachnida is a class of joint-legged invertebrate animals (arthropods), in the subphylum Chelicerata. Arachnida includes, among others, spiders, scorpions, ticks, mites, pseudoscorpions, harvestmen, camel spiders, whip spiders and vinegaroons.

Pseudoscorpions, also known as false scorpions or book scorpions, are small, scorpion-like arachnids belonging to the order Pseudoscorpiones, also known as Pseudoscorpionida or Chelonethida.

Amblypygi is an order of arachnid chelicerate arthropods also known as whip spiders or tailless whip scorpions. The name "amblypygid" means "blunt tail", a reference to a lack of the flagellum that is otherwise seen in whip scorpions. Amblypygids possess no silk glands or venomous fangs. They rarely bite if threatened, but can grab fingers with their pedipalps, resulting in thorn-like puncture injuries.

Schizomida, also known as sprickets or short-tailed whip-scorpions, is an order of arachnids, generally less than 5 millimetres (0.20 in) in length. The order is not yet widely studied. E. O. Wilson has identified schizomids as among the "groups of organisms that desperately need experts to work on them."

The Opiliones are an order of arachnids colloquially known as harvestmen, harvesters, harvest spiders, or daddy longlegs. As of April 2017, over 6,650 species of harvestmen have been discovered worldwide, although the total number of extant species may exceed 10,000. The order Opiliones includes five suborders: Cyphophthalmi, Eupnoi, Dyspnoi, Laniatores, and Tetrophthalmi, which were named in 2014.

Xiphosura is an order of arthropods related to arachnids. They are more commonly known as horseshoe crabs. They first appeared in the Hirnantian. Currently, there are only four living species. Xiphosura contains one suborder, Xiphosurida, and several stem-genera.

Opilioacaridae is the sole family of mites in the order Opilioacarida, made up of about 13 genera. The mites of this family are rare, large mites, and are widely considered primitive, as they retain six pairs of eyes, and abdominal segmentation. They have historically been considered separate from other mites belonging to Acariformes and Parasitiformes, but are now generally considered a subgroup of Parasitiformes based on molecular phylogenetics.
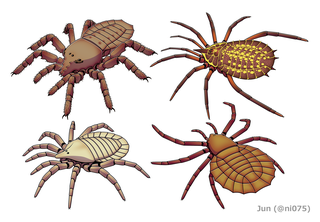
The order Trigonotarbida is a group of extinct arachnids whose fossil record extends from the late Silurian to the early Permian. These animals are known from several localities in Europe and North America, as well as a single record from Argentina. Trigonotarbids can be envisaged as spider-like arachnids, but without silk-producing spinnerets. They ranged in size from a few millimetres to a few centimetres in body length and had segmented abdomens (opisthosoma), with the dorsal exoskeleton (tergites) across the backs of the animals' abdomens, which were characteristically divided into three or five separate plates. Probably living as predators on other arthropods, some later trigonotarbid species were quite heavily armoured and protected themselves with spines and tubercles. About seventy species are currently known, with most fossils originating from the Carboniferous coal measures.

Tetrapulmonata is a non-ranked supra-ordinal clade of arachnids. It is composed of the extant orders Uropygi, Schizomida, Amblypygi and Araneae (spiders). It is the only supra-ordinal group of arachnids that is strongly supported in molecular phylogenetic studies. Two extinct orders are also placed in this clade, Haptopoda and Uraraneida. In 2016, a newly described fossil arachnid, Idmonarachne, was also included in the Tetrapulmonata; as of March 2016 it has not been assigned to an order.

Archaeidae, also known as assassin spiders and pelican spiders, is a spider family with about ninety described species in five genera. It contains small spiders, ranging from 2 to 8 millimetres long, that prey exclusively on other spiders. They are unusual in that they have "necks", ranging from long and slender to short and thick. The name "pelican spider" refers to these elongated jaws and necks used to catch their prey. Living species of Archaeidae occur in South Africa, Madagascar and Australia, with the sister family Mecysmaucheniidae occurring in southern South America and New Zealand.

The evolution of spiders has been ongoing for at least 380 million years. The group's origins lie within an arachnid sub-group defined by the presence of book lungs ; the arachnids as a whole evolved from aquatic chelicerate ancestors. More than 45,000 extant species have been described, organised taxonomically in 3,958 genera and 114 families. There may be more than 120,000 species. Fossil diversity rates make up a larger proportion than extant diversity would suggest with 1,593 arachnid species described out of 1,952 recognized chelicerates. Both extant and fossil species are described annually by researchers in the field. Major developments in spider evolution include the development of spinnerets and silk secretion.

Mongolarachne is an extinct genus of spiders placed in the monogeneric family Mongolarachnidae. The genus contains only one species, Mongolarachne jurassica, described in 2013, which is presently the largest fossilized spider on record. The type species was originally described as Nephila jurassica and placed in the living genus Nephila which contains the golden silk orb-weavers.
The Panther Mountain Formation is a geologic formation in New York. It preserves fossils dating back to the Devonian period. It is located in the counties of Albany, Madison, Oneida, Otsego, and Schoharie. It is well known for its fossil arthropods preserved as flattened cuticles, including Attercopus and Dracochela.
This list of fossil arthropods described in 2015 is a list of new taxa of trilobites, fossil insects, crustaceans, arachnids and other fossil arthropods of every kind that have been described during the year 2015. The list only includes taxa at the level of genus or species.

Pseudocellus is an arachnid genus in the order Ricinulei, first described by Norman Platnick in 1980. It is native to the Neotropics.

Pseudocellus pearsei is an arachnid species in the order Ricinulei. It occurs in caves in Yucatan, Mexico.
This list of fossil arthropods described in 2018 is a list of new taxa of trilobites, fossil insects, crustaceans, arachnids, and other fossil arthropods of every kind that were described during the year 2018, as well as other significant discoveries, and events related to arthropod paleontology that are scheduled to occur in the year 2018.

The subcapitulum, also known as infracapitulum, hypognathum or hipognatum, refers to the ventral part of the gnathosoma or the fusion of the palpal coxae and the labrum complex present in some arthropods on which the mouth, pedipalps, mouthparts and pharynx are generally located. It is delimited by the subcapitular apodeme, which separates it from the cheliceral frame.
Burmese amber is fossil resin dating to the early Late Cretaceous Cenomanian age recovered from deposits in the Hukawng Valley of northern Myanmar. It is known for being one of the most diverse Cretaceous age amber paleobiotas, containing rich arthropod fossils, along with uncommon vertebrate fossils and even rare marine inclusions. A mostly complete list of all taxa described up until 2018 can be found in Ross 2018; its supplement Ross 2019b covers most of 2019.



















