The lungs are the central organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. The pleurae, which are thin, smooth, and moist, serve to reduce friction between the lungs and chest wall during breathing, allowing for easy and effortless movements of the lungs.

The respiratory system is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies greatly, depending on the size of the organism, the environment in which it lives and its evolutionary history. In land animals, the respiratory surface is internalized as linings of the lungs. Gas exchange in the lungs occurs in millions of small air sacs; in mammals and reptiles, these are called alveoli, and in birds, they are known as atria. These microscopic air sacs have a very rich blood supply, thus bringing the air into close contact with the blood. These air sacs communicate with the external environment via a system of airways, or hollow tubes, of which the largest is the trachea, which branches in the middle of the chest into the two main bronchi. These enter the lungs where they branch into progressively narrower secondary and tertiary bronchi that branch into numerous smaller tubes, the bronchioles. In birds, the bronchioles are termed parabronchi. It is the bronchioles, or parabronchi that generally open into the microscopic alveoli in mammals and atria in birds. Air has to be pumped from the environment into the alveoli or atria by the process of breathing which involves the muscles of respiration.

A pulmonary alveolus, also known as an air sac or air space, is one of millions of hollow, distensible cup-shaped cavities in the lungs where pulmonary gas exchange takes place. Oxygen is exchanged for carbon dioxide at the blood–air barrier between the alveolar air and the pulmonary capillary. Alveoli make up the functional tissue of the mammalian lungs known as the lung parenchyma, which takes up 90 percent of the total lung volume.
Diffusing capacity of the lung (DL) measures the transfer of gas from air in the lung, to the red blood cells in lung blood vessels. It is part of a comprehensive series of pulmonary function tests to determine the overall ability of the lung to transport gas into and out of the blood. DL, especially DLCO, is reduced in certain diseases of the lung and heart. DLCO measurement has been standardized according to a position paper by a task force of the European Respiratory and American Thoracic Societies.
Dead space is the volume of air that is inhaled that does not take part in the gas exchange, because it either remains in the conducting airways or reaches alveoli that are not perfused or poorly perfused. It means that not all the air in each breath is available for the exchange of oxygen and carbon dioxide. Mammals breathe in and out of their lungs, wasting that part of the inhalation which remains in the conducting airways where no gas exchange can occur.

The respiratory tract is the subdivision of the respiratory system involved with the process of conducting air to the alveoli for the purposes of gas exchange in mammals. The respiratory tract is lined with respiratory epithelium as respiratory mucosa.

Aquatic respiration is the process whereby an aquatic organism exchanges respiratory gases with water, obtaining oxygen from oxygen dissolved in water and excreting carbon dioxide and some other metabolic waste products into the water.

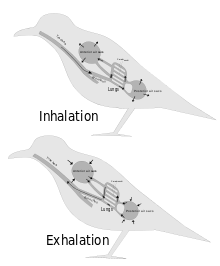
Inhalation is the process of drawing air or other gases into the respiratory tract, primarily for the purpose of bringing oxygen into the body. It is a fundamental physiological function in humans and many other organisms, essential for sustaining life. Inhalation is the first phase of breathing, allowing the exchange of oxygen and carbon dioxide between the body and the environment, vital for the body's metabolic processes. This article delves into the mechanics of inhalation, its significance in various contexts, and its potential impact on health.

Exhalation is the flow of the breath out of an organism. In animals, it is the movement of air from the lungs out of the airways, to the external environment during breathing. This happens due to elastic properties of the lungs, as well as the internal intercostal muscles which lower the rib cage and decrease thoracic volume. As the thoracic diaphragm relaxes during exhalation it causes the tissue it has depressed to rise superiorly and put pressure on the lungs to expel the air. During forced exhalation, as when blowing out a candle, expiratory muscles including the abdominal muscles and internal intercostal muscles generate abdominal and thoracic pressure, which forces air out of the lungs.
In physiology, respiration is the movement of oxygen from the outside environment to the cells within tissues, and the removal of carbon dioxide in the opposite direction to the surrounding environment.

Hypoxemia is an abnormally low level of oxygen in the blood. More specifically, it is oxygen deficiency in arterial blood. Hypoxemia has many causes, and often causes hypoxia as the blood is not supplying enough oxygen to the tissues of the body.
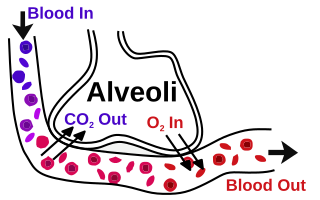
The blood–air barrier or air–blood barrier, exists in the gas exchanging region of the lungs. It exists to prevent air bubbles from forming in the blood, and from blood entering the alveoli. It is formed by the type I pneumocytes of the alveolar wall, the endothelial cells of the capillaries and the basement membrane between. The barrier is permeable to molecular oxygen, carbon dioxide, carbon monoxide and many other gases.
In respiratory physiology, the ventilation/perfusion ratio is a ratio used to assess the efficiency and adequacy of the ventilation-perfusion coupling and thus the matching of two variables:
A pulmonary shunt is the passage of deoxygenated blood from the right side of the heart to the left without participation in gas exchange in the pulmonary capillaries. It is a pathological condition that results when the alveoli of parts of the lungs are perfused with blood as normal, but ventilation fails to supply the perfused region. In other words, the ventilation/perfusion ratio of those areas is zero.
The Alveolar–arterial gradient, is a measure of the difference between the alveolar concentration (A) of oxygen and the arterial (a) concentration of oxygen. It is a useful parameter for narrowing the differential diagnosis of hypoxemia.

Breathing is the rhythmical process of moving air into and out of the lungs to facilitate gas exchange with the internal environment, mostly to flush out carbon dioxide and bring in oxygen.
Work of breathing (WOB) is the energy expended to inhale and exhale a breathing gas. It is usually expressed as work per unit volume, for example, joules/litre, or as a work rate (power), such as joules/min or equivalent units, as it is not particularly useful without a reference to volume or time. It can be calculated in terms of the pulmonary pressure multiplied by the change in pulmonary volume, or in terms of the oxygen consumption attributable to breathing.
Human physiology of underwater diving is the physiological influences of the underwater environment on the human diver, and adaptations to operating underwater, both during breath-hold dives and while breathing at ambient pressure from a suitable breathing gas supply. It, therefore, includes the range of physiological effects generally limited to human ambient pressure divers either freediving or using underwater breathing apparatus. Several factors influence the diver, including immersion, exposure to the water, the limitations of breath-hold endurance, variations in ambient pressure, the effects of breathing gases at raised ambient pressure, effects caused by the use of breathing apparatus, and sensory impairment. All of these may affect diver performance and safety.
In respiratory physiology, the oxygen cascade describes the flow of oxygen from air to mitochondria, where it is consumed in aerobic respiration to release energy. Oxygen flows from areas with high partial pressure of oxygen (PO2, also known as oxygen tension) to areas of lower PO2.

Ventilation-perfusion coupling is the relationship between ventilation and perfusion processes, which take place in the respiratory system and the cardiovascular system. Ventilation is the movement of gas during breathing, and perfusion is the process of pulmonary blood circulation, which delivers oxygen to body tissues. Anatomically, the lung structure, alveolar organization, and alveolar capillaries contribute to the physiological mechanism of ventilation and perfusion. Ventilation-perfusion coupling maintains a constant ventilation/perfusion ratio near 0.8 on average, while the regional variation exists within the lungs due to gravity. When the ratio gets above or below 0.8, it is considered abnormal ventilation-perfusion coupling, also known as a ventilation–perfusion mismatch. Lung diseases, cardiac shunts, and smoking can cause a ventilation-perfusion mismatch that results in significant symptoms and diseases, which can be treated through treatments like bronchodilators and oxygen therapy.