Related Research Articles

Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Neutron activation analysis (NAA) is a nuclear process used for determining the concentrations of elements in many materials. NAA allows discrete sampling of elements as it disregards the chemical form of a sample, and focuses solely on atomic nuclei. The method is based on neutron activation and thus requires a neutron source. The sample is bombarded with neutrons, causing its constituent elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element have long been studied and determined. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the various elements within it. A particular advantage of this technique is that it does not destroy the sample, and thus has been used for the analysis of works of art and historical artifacts. NAA can also be used to determine the activity of a radioactive sample.

Environmental remediation is the cleanup of hazardous substances dealing with the removal, treatment and containment of pollution or contaminants from environmental media such as soil, groundwater, sediment. Remediation may be required by regulations before development of land revitalization projects. Developers who agree to voluntary cleanup may be offered incentives under state or municipal programs like New York State's Brownfield Cleanup Program. If remediation is done by removal the waste materials are simply transported off-site for disposal at another location. The waste material can also be contained by physical barriers like slurry walls. The use of slurry walls is well-established in the construction industry. The application of (low) pressure grouting, used to mitigate soil liquefaction risks in San Francisco and other earthquake zones, has achieved mixed results in field tests to create barriers, and site-specific results depend upon many variable conditions that can greatly impact outcomes.

Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.
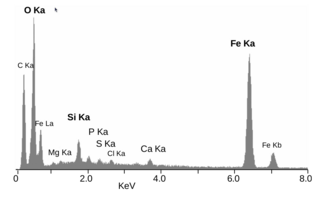
Energy-dispersive X-ray spectroscopy, sometimes called energy dispersive X-ray analysis or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum. The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument.

Caesium hydroxide is a strong base containing the highly reactive alkali metal caesium, much like the other alkali metal hydroxides such as sodium hydroxide and potassium hydroxide. It is the strongest of the five alkali metal hydroxides. Fused Caesium hydroxide dissolves glass by attacking silica framework and it has applications in bringing glass samples into a solution for analytical purposes in commercial glass industry and defense waste processing facility. The melting process is carried out in a nickel or zirconium crucible. Caesium hydroxide fusion at 750°C produces complete dissolution of glass pellets.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

Sonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seaweeds. Ultrasonic frequencies (> 20 kHz) are usually used, leading to the process also being known as ultrasonication or ultra-sonication.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
Solid phase microextraction, or SPME, is a solid phase extraction sampling technique that involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent), which extracts different kinds of analytes from different kinds of media, that can be in liquid or gas phase. The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.
Environmental monitoring describes the processes and activities that need to take place to characterize and monitor the quality of the environment. Environmental monitoring is used in the preparation of environmental impact assessments, as well as in many circumstances in which human activities carry a risk of harmful effects on the natural environment. All monitoring strategies and programs have reasons and justifications which are often designed to establish the current status of an environment or to establish trends in environmental parameters. In all cases, the results of monitoring will be reviewed, analyzed statistically, and published. The design of a monitoring program must therefore have regard to the final use of the data before monitoring starts.
Bioanalysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics and biotics in biological systems.
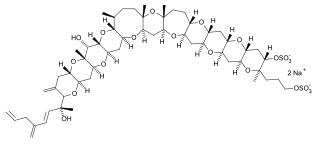
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.
Water chemistry analyses are carried out to identify and quantify the chemical components and properties of water samples. The type and sensitivity of the analysis depends on the purpose of the analysis and the anticipated use of the water. Chemical water analysis is carried out on water used in industrial processes, on waste-water stream, on rivers and stream, on rainfall and on the sea. In all cases the results of the analysis provides information that can be used to make decisions or to provide re-assurance that conditions are as expected. The analytical parameters selected are chosen to be appropriate for the decision making process or to establish acceptable normality. Water chemistry analysis is often the groundwork of studies of water quality, pollution, hydrology and geothermal waters. Analytical methods routinely used can detect and measure all the natural elements and their inorganic compounds and a very wide range of organic chemical species using methods such as gas chromatography and mass spectrometry. In water treatment plants producing drinking water and in some industrial processes using products with distinctive taste and odours, specialised organoleptic methods may be used to detect smells at very low concentrations.

Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term washing may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired compound.

Extractive electrospray ionization (EESI) is a spray-type, ambient ionization source in mass spectrometry that uses two colliding aerosols, one of which is generated by electrospray. In standard EESI, syringe pumps provide the liquids for both an electrospray and a sample spray. In neutral desorption EESI (ND-EESI), the liquid for the sample aerosol is provided by a flow of nitrogen.
Ion suppression in LC-MS and LC-MS/MS refers to reduced detector response, or signal:noise as a manifested effect of competition for ionisation efficiency in the ionisation source, between the analyte(s) of interest and other endogenous or exogenous species which have not been removed from the sample matrix during sample preparation. Ion suppression is not strictly a problem unless interfering compounds elute at the same time as the analyte of interest. In cases where ion suppressing species do co-elute with an analyte, the effects on the important analytical parameters including precision, accuracy and limit of detection can be extensive, severely limiting the validity of an assay's results.
Electro membrane extraction, or EME, is a miniaturized liquid-liquid extraction technique developed for sample preparation of aqueous samples prior to analysis by chromatography, electrophoresis, mass spectrometry, and related techniques in analytical chemistry. EME involves the use of a small supported liquid membrane (SLM) sustained in the wall of a porous hollow fiber, and application of an electrical field across the SLM.
Single-drop microextraction (SDME) is a sample preparation technique in chemical test or analytical chemistry. SDME uses only a single drop of solvent to isolate and preconcentrate analytes from a sample matrix. The extremely low solvent use of SDME makes it cost-effective and less harmful to the environment, subscribing to the principles of green analytical chemistry.
References
- "Sample Preparation" . Retrieved 2007-11-09.