
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Fourier-transform spectroscopy is a measurement technique whereby spectra are collected based on measurements of the coherence of a radiative source, using time-domain or space-domain measurements of the radiation, electromagnetic or not. It can be applied to a variety of types of spectroscopy including optical spectroscopy, infrared spectroscopy, nuclear magnetic resonance (NMR) and magnetic resonance spectroscopic imaging (MRSI), mass spectrometry and electron spin resonance spectroscopy.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state. The photon energy of the emitted photons is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.

Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.

A monochromator is an optical device that transmits a mechanically selectable narrow band of wavelengths of light or other radiation chosen from a wider range of wavelengths available at the input. The name is from the Greek roots mono-, "single", and chroma, "colour", and the Latin suffix -ator, denoting an agent.

X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray radiation.
A spectroradiometer is a light measurement tool that is able to measure both the wavelength and amplitude of the light emitted from a light source. Spectrometers discriminate the wavelength based on the position the light hits at the detector array allowing the full spectrum to be obtained with a single acquisition. Most spectrometers have a base measurement of counts which is the un-calibrated reading and is thus impacted by the sensitivity of the detector to each wavelength. By applying a calibration, the spectrometer is then able to provide measurements of spectral irradiance, spectral radiance and/or spectral flux. This data is also then used with built in or PC software and numerous algorithms to provide readings or Irradiance (W/cm2), Illuminance, Radiance (W/sr), Luminance (cd), Flux, Chromaticity, Color Temperature, Peak and Dominant Wavelength. Some more complex spectrometer software packages also allow calculation of PAR μmol/m2/s, Metamerism, and candela calculations based on distance and include features like 2- and 20-degree observer, baseline overlay comparisons, transmission and reflectance.

The NASA Infrared Telescope Facility is a 3-meter (9.8 ft) telescope optimized for use in infrared astronomy and located at the Mauna Kea Observatory in Hawaii. It was first built to support the Voyager missions and is now the US national facility for infrared astronomy, providing continued support to planetary, solar neighborhood, and deep space applications. The IRTF is operated by the University of Hawaii under a cooperative agreement with NASA. According to the IRTF's time allocation rules, at least 50% of the observing time is devoted to planetary science.

An imaging spectrometer is an instrument used in hyperspectral imaging and imaging spectroscopy to acquire a spectrally-resolved image of an object or scene, often referred to as a datacube due to the three-dimensional representation of the data. Two axes of the image correspond to vertical and horizontal distance and the third to wavelength. The principle of operation is the same as that of the simple spectrometer, but special care is taken to avoid optical aberrations for better image quality.

In optics, a dispersive prism is an optical prism that is used to disperse light, that is, to separate light into its spectral components. Different wavelengths (colors) of light will be deflected by the prism at different angles. This is a result of the prism material's index of refraction varying with wavelength (dispersion). Generally, longer wavelengths (red) undergo a smaller deviation than shorter wavelengths (blue). The dispersion of white light into colors by a prism led Sir Isaac Newton to conclude that white light consisted of a mixture of different colors.

The Cosmic Origins Spectrograph (COS) is a science instrument that was installed on the Hubble Space Telescope during Servicing Mission 4 (STS-125) in May 2009. It is designed for ultraviolet (90–320 nm) spectroscopy of faint point sources with a resolving power of ≈1,550–24,000. Science goals include the study of the origins of large scale structure in the universe, the formation and evolution of galaxies, and the origin of stellar and planetary systems and the cold interstellar medium. COS was developed and built by the Center for Astrophysics and Space Astronomy (CASA-ARL) at the University of Colorado at Boulder and the Ball Aerospace and Technologies Corporation in Boulder, Colorado.
A multivariate optical element (MOE), is the key part of a multivariate optical computer; an alternative to conventional spectrometry for the chemical analysis of materials.

The Extreme Ultraviolet Explorer was a NASA space telescope for ultraviolet astronomy. EUVE was a part of NASA's Explorer spacecraft series. Launched on 7 June 1992. With instruments for ultraviolet (UV) radiation between wavelengths of 7 and 76 nm, the EUVE was the first satellite mission especially for the short-wave ultraviolet range. The satellite compiled an all-sky survey of 801 astronomical targets before being decommissioned on 31 January 2001.

An echelle grating is a type of diffraction grating characterised by a relatively low groove density, but a groove shape which is optimized for use at high incidence angles and therefore in high diffraction orders. Higher diffraction orders allow for increased dispersion (spacing) of spectral features at the detector, enabling increased differentiation of these features. Echelle gratings are, like other types of diffraction gratings, used in spectrometers and similar instruments. They are most useful in cross-dispersed high resolution spectrographs, such as HARPS, PARAS, and numerous other astronomical instruments.

Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time.

Modern spectroscopy in the Western world started in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word spectrum to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum, which now bear his name although several of them were observed earlier by Wollaston.

The NIRSpec is one of the four scientific instruments flown on the James Webb Space Telescope (JWST). The JWST is the follow-on mission to the Hubble Space Telescope (HST) and is developed to receive more information about the origins of the universe by observing infrared light from the first stars and galaxies. In comparison to HST, its instruments will allow looking further back in time and will study the so-called Dark Ages during which the universe was opaque, about 150 to 800 million years after the Big Bang.

A spectrometer is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe.
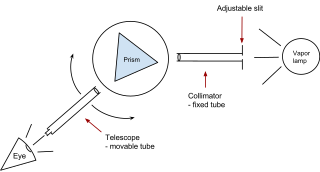
A prism spectrometer is an optical spectrometer which uses a dispersive prism as its dispersive element. The prism refracts light into its different colors (wavelengths). The dispersion occurs because the angle of refraction is dependent on the refractive index of the prism's material, which in turn is slightly dependent on the wavelength of light that is traveling through it.
























