
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
In chemistry, a nitrene or imene is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reactions. The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and in fact rarely isolated. Diradicals are even-electron molecules but have one fewer bond than the number permitted by the octet rule.

Tris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]2+ 2Cl−. This polypyridine complex is a red crystalline salt obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.

Atomic carbon, systematically named carbon and λ0-methane, is a colourless gaseous inorganic chemical with the chemical formula C. It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation.
In organic chemistry, the di-π-methane rearrangement is the photochemical rearrangement of a molecule that contains two π-systems separated by a saturated carbon atom. In the aliphatic case, this molecules is a 1,4-diene; in the aromatic case, an allyl-substituted arene. The reaction forms (respectively) an ene- or aryl-substituted cyclopropane. Formally, it amounts to a 1,2 shift of one ene group or the aryl group, followed by bond formation between the lateral carbons of the non-migrating moiety:
In chemistry, bond cleavage, or bond fission, is the splitting of chemical bonds. This can be generally referred to as dissociation when a molecule is cleaved into two or more fragments.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Ethylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. It is an oxide of carbon, and can be described as the carbon-carbon covalent dimer of carbon monoxide. It can also be thought of as the dehydrated form of glyoxylic acid, or a ketone of ethenone H2C=C=O.

Phosphinidenes are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties. In the last few decades, several strategies have been employed to stabilize phosphinidenes, and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.
In organic chemistry, enone–alkene cycloadditions are a version of the [2+2] cycloaddition This reaction involves an enone and alkene as substrates. Although the concerted photochemical [2+2] cycloaddition is allowed, the reaction between enones and alkenes is stepwise and involves discrete diradical intermediates.

9-Fluorenylidene is an aryl carbene derived from the bridging methylene group of fluorene. Fluorenylidene has the unusual property that the triplet ground state is only 1.1 kcal/mol lower in energy than the singlet state. For this reason, fluorenylidene has been studied extensively in organic chemistry.
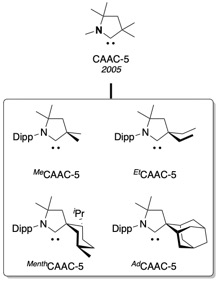
In chemistry, cyclic(alkyl)(amino)carbenes (CAACs) are a family of stable singlet carbene ligands developed by the research group of Guy Bertrand in 2005 at UC Riverside. In marked contrast with the popular N-heterocyclic carbenes (NHCs) which possess two "amino" substituents adjacent to the carbene center, CAACs possess one "amino" substituent and an sp3 carbon atom "alkyl". This specific configuration makes the CAACs very good σ-donors and π-acceptors when compared to NHCs. Moreover the reduced heteroatom stabilization of the carbene center in CAACs versus NHCs also gives rise to a smaller ΔEST.

Triplet-triplet annihilation (TTA) is an energy transfer mechanism where two molecules in their triplet excited states interact to form a ground state molecule and an excited molecule in its singlet state. This mechanism is example of Dexter energy transfer mechanism. In triplet-triplet annihilation, one molecule transfers its excited state energy to the second molecule, resulting in the first molecule returning to its ground state and the second molecule being promoted to a higher excited singlet state.

Boraacenes are polycyclic aromatic hydrocarbons containing at least one boron atom. Structurally, they are related to acenes, linearly fused benzene rings. However, the boron atom is electron deficient and may act as a Lewis Acid when compared to carbon. This results in slightly less negative charge within the ring, smaller HOMO-LUMO gaps, as well as differences in redox chemistry when compared to their acene analogues. When incorporated into acenes, Boron maintains the planarity and aromaticity of carbon acenes, while adding an empty p-orbital, which can be utilized for the fine tuning of organic semiconductor band gaps. Due to this empty p orbital, however, it is also highly reactive when exposed to nucleophiles like water or normal atmosphere, as it will readily be attacked by oxygen, which must be addressed to maintain its stability.












