Organic electronics is a field of materials science concerning the design, synthesis, characterization, and application of organic molecules or polymers that show desirable electronic properties such as conductivity. Unlike conventional inorganic conductors and semiconductors, organic electronic materials are constructed from organic (carbon-based) molecules or polymers using synthetic strategies developed in the context of organic chemistry and polymer chemistry.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
Antiaromaticity is a characteristic of a cyclic molecule with a π electron system that has higher energy due to the presence of 4n delocalised electrons in it. Unlike aromatic compounds, which follow Hückel's rule and are highly stable, antiaromatic compounds are highly unstable and highly reactive. To avoid the instability of antiaromaticity, molecules may change shape, becoming non-planar and therefore breaking some of the π interactions. In contrast to the diamagnetic ring current present in aromatic compounds, antiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas MC≡CH and MC≡CM, where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure RC−CM. Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce.

An organic field-effect transistor (OFET) is a field-effect transistor using an organic semiconductor in its channel. OFETs can be prepared either by vacuum evaporation of small molecules, by solution-casting of polymers or small molecules, or by mechanical transfer of a peeled single-crystalline organic layer onto a substrate. These devices have been developed to realize low-cost, large-area electronic products and biodegradable electronics. OFETs have been fabricated with various device geometries. The most commonly used device geometry is bottom gate with top drain and source electrodes, because this geometry is similar to the thin-film silicon transistor (TFT) using thermally grown SiO2 as gate dielectric. Organic polymers, such as poly(methyl-methacrylate) (PMMA), can also be used as dielectric. One of the benefits of OFETs, especially compared with inorganic TFTs, is their unprecedented physical flexibility, which leads to biocompatible applications, for instance in the future health care industry of personalized biomedicines and bioelectronics.

Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.

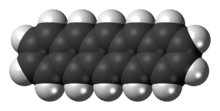
Hexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility.

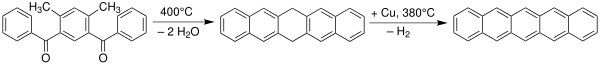
Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings. This compound has long been pursued by chemists because of its potential interest in electronic applications and was first synthesized but not cleanly isolated in 2006. Heptacene was finally fully characterized in bulk by researchers in Germany and the United States in 2017.

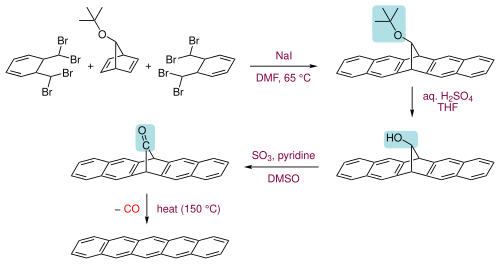
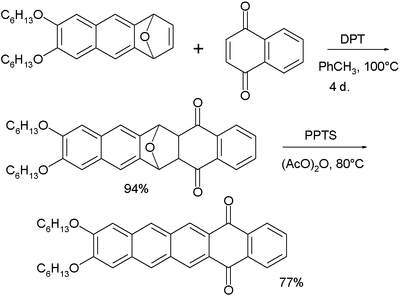
In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene rings which have been linearly fused. They follow the general molecular formula C4n+2H2n+4.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.
The Buchner ring expansion is a two-step organic C-C bond forming reaction used to access 7-membered rings. The first step involves formation of a carbene from ethyl diazoacetate, which cyclopropanates an aromatic ring. The ring expansion occurs in the second step, with an electrocyclic reaction opening the cyclopropane ring to form the 7-membered ring.
Singlet fission is a spin-allowed process, unique to molecular photophysics, whereby one singlet excited state is converted into two triplet states. The phenomenon has been observed in molecular crystals, aggregates, disordered thin films, and covalently-linked dimers, where the chromophores are oriented such that the electronic coupling between singlet and the double triplet states is large. Being spin allowed, the process can occur very rapidly and out-compete radiative decay thereby producing two triplets with very high efficiency. The process is distinct from intersystem crossing, in that singlet fission does not involve a spin flip, but is mediated by two triplets coupled into an overall singlet. It has been proposed that singlet fission in organic photovoltaic devices could improve the photoconversion efficiencies.

Phenacenes are a class of organic compounds consisting of fused aromatic rings. They are polycyclic aromatic hydrocarbons, related to acenes and helicenes from which they differ by the arrangement of the fused rings.
Diketopyrrolopyrroles (DPPs) are organic dyes and pigments based on the heterocyclic dilactam 2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione, widely used in optoelectronics. DPPs were initially used as pigments in the painting industry due to their high resistance to photodegradation. More recently, DPP derivatives have been also investigated as promising fluorescent dyes for bioimaging applications, as well as components of materials for use in organic electronics.

Contorted aromatics or more precisely contorted polycyclic aromatic hydrocarbons are polycyclic aromatic hydrocarbons (PAHs) in which the fused aromatic molecules deviate from the usual planarity.