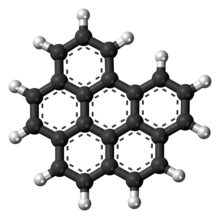
Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising seven peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites (more commonly known as BPDE) react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.
Substances, mixtures and exposure circumstances in this list have been classified by the International Agency for Research on Cancer (IARC) as group 3: The agent is not classifiable as to its carcinogenicity to humans. This category is used most commonly for agents, mixtures and exposure circumstances for which the evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.
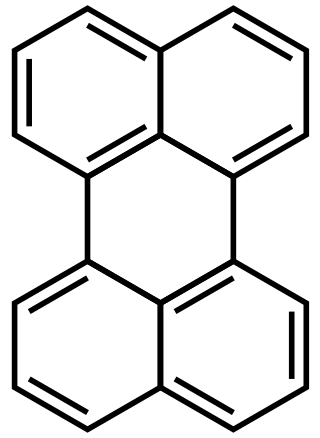
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.
Neoflavonoids are a class of polyphenolic compounds. While flavonoids have the 2-phenylchromen-4-one backbone, neoflavonoids have the 4-phenylchromen backbone with no hydroxyl group substitution at position 2.
The Water Supply Water Quality Regulations 1989 are regulations imposed on the England and Wales Water industry by statutory instrument. The regulations were signed jointly by Peter Walker, Secretary of State for Wales and Michael Howard who, as Minister for Water and Planning, was responsible for implementing water privatization in England and Wales during 1988/89. Most of the measures came into force on 1 September 1989, and the rest on 1 January 1990. The regulations have been superseded by several later instruments.

A benzopyrene is an organic compound with the formula C20H12. Structurally speaking, the colorless isomers of benzopyrene are pentacyclic hydrocarbons and are fusion products of pyrene and a phenylene group. Two isomeric species of benzopyrene are benzo[a]pyrene and the less common benzo[e]pyrene. They belong to the chemical class of polycyclic aromatic hydrocarbons.
The molecular formula C22H12 (molar mass: 276.33 g/mol, exact mass: 276.0939 u) may refer to:
The molecular formula C20H12 may refer to:

Benzo[k]fluoranthene is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it forms pale yellow needles or crystals, and is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo(a)fluoranthene, benzo(b)fluoranthene, benzo(e)fluoranthene, and benzo(j)fluoranthene.

Methysticin is one of the six major kavalactones found in the kava plant. Research suggests that methysticin and the related compound dihydromethysticin have CYP1A1 inducing effects which may be responsible for their toxicity. Additionally, methysticin has been shown to potentiate GABAA receptor activity, contributing to the overall anxiolytic profile of the kava plant.

Isocoumarin is a lactone, a type of natural organic compound.

A rylene dye is a dye based on the rylene framework of naphthalene units linked in peri-positions. In homologues additional naphthalene units are added, forming compounds — or poly(peri-naphthalene)s — such as perylene, terrylene and quarterrylene.

Olympicene is an organic carbon-based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings.

Pigment Violet 29 is an organic compound that is used as a pigment and vat dye. Its colour is dark red purple, or bordeaux.
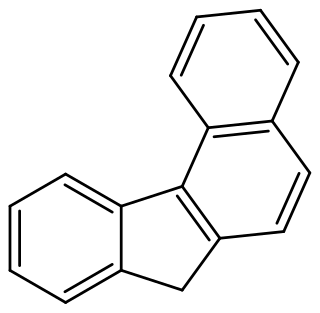
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
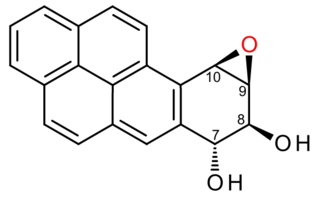
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.

Horse Eye Sewer is a small, 4.8-kilometre (3.0 mi) long stream (brook) of the Pevensey Levels in the Wealden District of East Sussex, England. A tributary to Hurst Haven, Horse Eye Sewer acts as a drainage ditch for several minor streams, many of which are unnamed.