Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis (dandruff). It may be used in combination with ultraviolet light therapy. Industrially it is a railroad tie preservative and used in the surfacing of roads. Coal tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government.
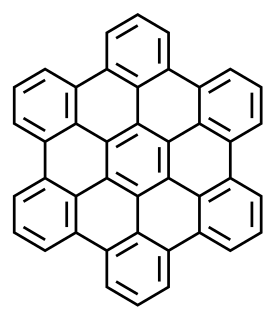
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Benzo[a]pyrene (BaP or B[a]P) is a polycyclic aromatic hydrocarbon and the result of incomplete combustion of organic matter at temperatures between 300 °C (572 °F) and 600 °C (1,112 °F). The ubiquitous compound can be found in coal tar, tobacco smoke and many foods, especially grilled meats. The substance with the formula C20H12 is one of the benzopyrenes, formed by a benzene ring fused to pyrene. Its diol epoxide metabolites (more commonly known as BPDE) react with and bind to DNA, resulting in mutations and eventually cancer. It is listed as a Group 1 carcinogen by the IARC. In the 18th century a scrotal cancer of chimney sweepers, the chimney sweeps' carcinoma, was already known to be connected to soot.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.

Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is C16H10. This yellow solid is the smallest peri-fused PAH. Pyrene forms during incomplete combustion of organic compounds.

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.
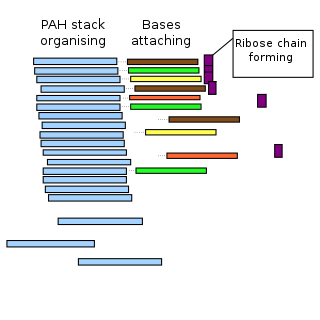
The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.

Cytochrome P450, family 1, subfamily A, polypeptide 1 is a protein that in humans is encoded by the CYP1A1 gene. The protein is a member of the cytochrome P450 superfamily of enzymes.
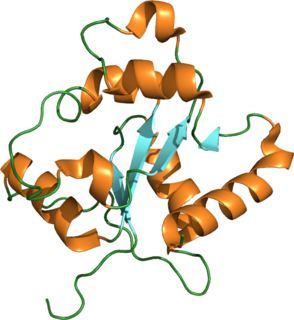
Toll-like receptor 2 also known as TLR2 is a protein that in humans is encoded by the TLR2 gene. TLR2 has also been designated as CD282. TLR2 is one of the toll-like receptors and plays a role in the immune system. TLR2 is a membrane protein, a receptor, which is expressed on the surface of certain cells and recognizes foreign substances and passes on appropriate signals to the cells of the immune system.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5–6 mg/kg.
Mycobacterium pyrenivorans is a scotochromogenic, rapidly growing mycobacterium, first isolated from an enrichment culture obtained from soil that was highly contaminated with polycyclic aromatic hydrocarbons (PAHs). The soil sample was collected on the site of a former coking plant at Ubach-Palenberg, Germany. Etymology: pyrenivorans; digesting pyrene.

Benzo[ghi]perylene is a polycyclic aromatic hydrocarbon with the chemical formula C22H12.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.

Methysticin is one of the six major kavalactones found in the kava plant. Research suggests that methysticin and the related compound dihydromethysticin have CYP1A1 inducing effects which may be responsible for their toxicity.

Dibenzopyrenes are a group of high molecular weight polycyclic aromatic hydrocarbons with the molecular formula C24H14. There are five isomers of dibenzopyrene which differ by the arrangement of aromatic rings: dibenzo[a,e]pyrene, dibenzo[a,h]pyrene, dibenzo[a,i]pyrene, dibenzo[a,l]pyrene, and dibenzo[e,l]pyrene.

2-Methylnaphthalene is a polycyclic aromatic hydrocarbon (PAH).
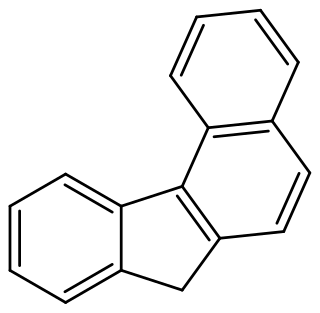
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
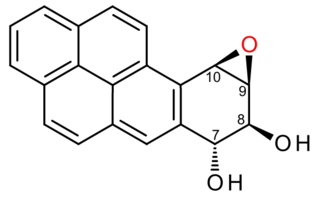
(+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide is an organic compound with molecular formula C20H14O3. It is a metabolite and derivative of benzo[a]pyrene (found in tobacco smoke) as a result of oxidation to include hydroxyl and epoxide functionalities. (+)-Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide binds to the N2 atom of a guanine nucleobase in DNA, distorting the double helix structure by intercalation of the pyrene moiety between base pairs through π-stacking. The carcinogenic properties of tobacco smoking are attributed in part to this compound binding and inactivating the tumor suppression ability of certain genes, leading to genetic mutations and potentially to cancer.
Gordonia sp. nov. Q8 is a bacterium in the phylum of Actinomycetota. It was discovered in 2017 as one of eighteen new species isolated from the Jiangsu Wei5 oilfield in East China with the potential for bioremediation. Strain Q8 is rod-shaped and gram-positive with dimensions 1.0–4.0 μm × 0.5–1.2 μm and an optimal growth temperature of 40 °C. Phylogenetically, it is most closely related to Gordonia paraffinivorans and Gordonia alkaliphila, both of which are known bioremediators. Q8 was assigned as a novel species based on a <70% ratio of DNA homology with other Gordonia bacteria.

Indeno[1,2,3-cd]pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples. One of these 16 is Indeno[1,2,3-cd]pyrene (IP). IP is the combination of an indeno molecule and a pyrene molecule with a fluoranthene network. In 1962, the National Cancer Institute reported that indeno[1,2,3-cd]pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.
![Chemical structure of benzo[a]pyrene Benzo-a-pyrene.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/f/fa/Benzo-a-pyrene.svg/200px-Benzo-a-pyrene.svg.png)
![Chemical structure of benzo[e]pyrene Benzo(e)pyrene.png](http://upload.wikimedia.org/wikipedia/commons/thumb/8/82/Benzo%28e%29pyrene.png/125px-Benzo%28e%29pyrene.png)
















