
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.
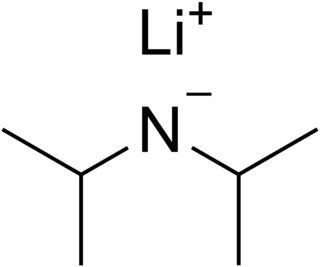
Lithium diisopropylamide is a chemical compound with the molecular formula LiN(CH 2)2. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

The Holton Taxol total synthesis, published by Robert A. Holton and his group at Florida State University in 1994, was the first total synthesis of Taxol.
Chromohalobacter beijerinckii is a motile, rod-like, salt-loving, Gram-negative soil bacterium, 0.4–0.6 μm by 1.8–2.5 μm.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.

Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C6(OH)12 or (C 2)6. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone.
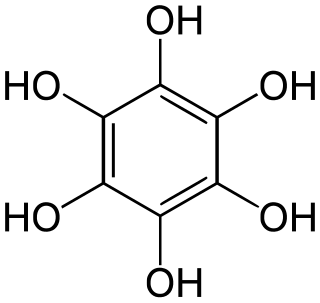
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C
6H
6O
6 or C
6(OH)
6. It is a six-fold phenol of benzene. The product is also called hexaphenol, but this name has been used also for other substances.
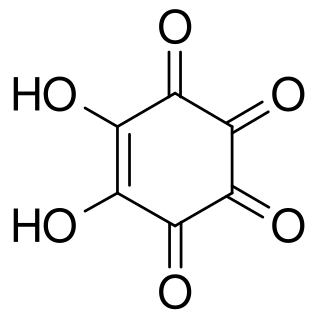
Rhodizonic acid is a chemical compound with formula H2C6O6 or (CO)4(COH)2. It can be seen as a twofold enol and fourfold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone.

Acetylenedicarboxylic acid or butynedioic acid is an organic compound with the formula H2C4O4 or HO−C(=O)−C≡C−C(=O)−OH. It is a crystalline solid that is soluble in diethyl ether.

Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula C
10O
10. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.

Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.
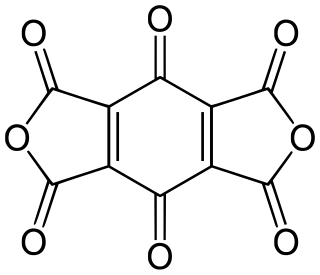
Benzoquinonetetracarboxylic dianhydride is an organic compound with formula C
10O
8 which can be seen as the result of removing two molecules of water H
2O from benzoquinonetetracarboxylic acid.
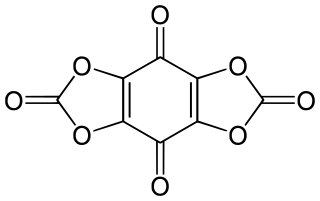
Tetrahydroxy-1,4-benzoquinone biscarbonate is a chemical compound, an oxide of carbon with formula C
8O
8. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two carbonate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and carbonic acid.
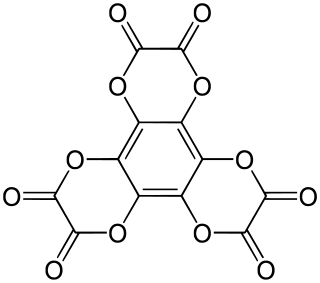
Hexahydroxybenzene trisoxalate is a chemical compound, an oxide of carbon with formula C
12O
12. Its molecule consists of a benzene core with the six hydrogen atoms replaced by three oxalate groups. It can be seen as a sixfold ester of benzenehexol and oxalic acid.

1,4-Naphthoquinone or para-naphthoquinone is a quinone derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.




















