
In thermodynamics and engineering, a heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity. When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.
In thermal fluid dynamics, the Nusselt number is the ratio of convective to conductive heat transfer at a boundary in a fluid. Convection includes both advection and diffusion (conduction). The conductive component is measured under the same conditions as the convective but for a hypothetically motionless fluid. It is a dimensionless number, closely related to the fluid's Rayleigh number.
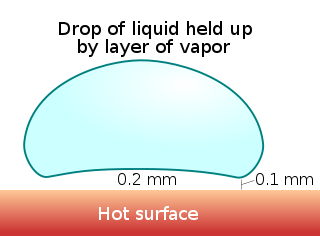
The Leidenfrost effect is a physical phenomenon in which a liquid, close to a surface that is significantly hotter than the liquid's boiling point, produces an insulating vapor layer that keeps the liquid from boiling rapidly. Because of this repulsive force, a droplet hovers over the surface, rather than making physical contact with it. The effect is named after the German doctor Johann Gottlob Leidenfrost, who described it in A Tract About Some Qualities of Common Water.
The Biot number (Bi) is a dimensionless quantity used in heat transfer calculations, named for the eighteenth-century French physicist Jean-Baptiste Biot (1774–1862). The Biot number is the ratio of the thermal resistance for conduction inside a body to the resistance for convection at the surface of the body. This ratio indicates whether the temperature inside a body varies significantly in space when the body is heated or cooled over time by a heat flux at its surface.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
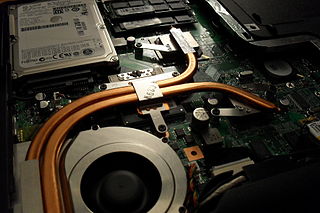
A heat pipe is a heat-transfer device that employs phase transition to transfer heat between two solid interfaces.
In thermodynamics, the heat transfer coefficient or film coefficient, or film effectiveness, is the proportionality constant between the heat flux and the thermodynamic driving force for the flow of heat. It is used in calculating the heat transfer, typically by convection or phase transition between a fluid and a solid. The heat transfer coefficient has SI units in watts per square meter per kelvin (W/m²K).
A coolant is a substance, typically liquid, that is used to reduce or regulate the temperature of a system. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, chemically inert and neither causes nor promotes corrosion of the cooling system. Some applications also require the coolant to be an electrical insulator.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
In the study of heat transfer, critical heat flux (CHF) is the heat flux at which boiling ceases to be an effective form of transferring heat from a solid surface to a liquid.

A reaction calorimeter is a calorimeter that measures the amount of energy released (exothermic) or absorbed (endothermic) by a chemical reaction. These measurements provide a more accurate picture of such reactions.
Micro heat exchangers,Micro-scale heat exchangers, or microstructured heat exchangers are heat exchangers in which fluid flows in lateral confinements with typical dimensions below 1 mm. The most typical such confinement are microchannels, which are channels with a hydraulic diameter below 1 mm. Microchannel heat exchangers can be made from metal or ceramic.
In fluid thermodynamics, nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature by between 10 and 30 °C. The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling.
In convective heat transfer, the Churchill–Bernstein equation is used to estimate the surface averaged Nusselt number for a cylinder in cross flow at various velocities. The need for the equation arises from the inability to solve the Navier–Stokes equations in the turbulent flow regime, even for a Newtonian fluid. When the concentration and temperature profiles are independent of one another, the mass-heat transfer analogy can be employed. In the mass-heat transfer analogy, heat transfer dimensionless quantities are replaced with analogous mass transfer dimensionless quantities.
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others.
Heat transfer enhancement is the process of increasing the effectiveness of heat exchangers. This can be achieved when the heat transfer power of a given device is increased or when the pressure losses generated by the device are reduced. A variety of techniques can be applied to this effect, including generating strong secondary flows or increasing boundary layer turbulence.
The removal of heat from nuclear reactors is an essential step in the generation of energy from nuclear reactions. In nuclear engineering there are a number of empirical or semi-empirical relations used for quantifying the process of removing heat from a nuclear reactor core so that the reactor operates in the projected temperature interval that depends on the materials used in the construction of the reactor. The effectiveness of removal of heat from the reactor core depends on many factors, including the cooling agents used and the type of reactor. Common liquid coolants for nuclear reactors include: deionized water, heavy water, the lighter alkaline metals, lead or lead-based eutectic alloys like lead-bismuth, and NaK, a eutectic alloy of sodium and potassium. Gas cooled reactors operate with coolants like carbon dioxide, helium or nitrogen but some very low powered research reactors have even been air-cooled with Chicago Pile 1 relying on natural convection of the surrounding air to remove the negligible thermal power output. There is ongoing research into using supercritical fluids as reactor coolants but thus far neither the supercritical water reactor nor a reactor cooled with supercritical Carbon Dioxide nor any other kind of supercritical-fluid-cooled reactor has ever been built.
Pillow-plate heat exchangers are a class of fully welded heat exchanger design, which exhibit a wavy, “pillow-shaped” surface formed by an inflation process. Compared to more conventional equipment, such as shell and tube and plate and frame heat exchangers, pillow plates are a quite young technology. Due to their geometric flexibility, they are used as well as “plate-type” heat exchangers and as jackets for cooling or heating of vessels. Pillow plate equipment is currently experiencing increased attention and implementation in process industry.
[6] Mukesh Kumar Sahu, Manjeet Kharub, Mahalingam Murugesan Matheswaran. “Nusselt number and friction factor correlation development for arc‑shape apex upstream artificial roughness in solar air heater.” Environmental Science and Pollution Research. Vol. 26, Pages- 65025–65042, 2022.







