Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.

Brazing is a metal-joining process in which two or more metal items are joined by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.

A heat sink is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is dissipated away from the device, thereby allowing regulation of the device's temperature. In computers, heat sinks are used to cool CPUs, GPUs, and some chipsets and RAM modules. Heat sinks are used with high-power semiconductor devices such as power transistors and optoelectronics such as lasers and light-emitting diodes (LEDs), where the heat dissipation ability of the component itself is insufficient to moderate its temperature.

Computer cooling is required to remove the waste heat produced by computer components, to keep components within permissible operating temperature limits. Components that are susceptible to temporary malfunction or permanent failure if overheated include integrated circuits such as central processing units (CPUs), chipsets, graphics cards, and hard disk drives.

Galinstan (R) is a brand name for an alloy composed of gallium, indium, and tin which melts at −19 °C (−2 °F) and is thus liquid at room temperature. However, it is not a eutectic alloy but a near eutectic alloy. In scientific literature, galinstan is also used as an acronym denoting the eutectic composition of the alloy of Ga-In-Sn, which melts at around +11 °C (52 °F). The composition of both alloys is roughly the same, albeit the Galinstan (R), a company's commercial technical product, likely has added flux to improve flowability, to reduce melting temperature, and to reduce surface tension. Thus, the physical properties of the Galinstan (R) and the pure eutectic alloy EGaInSn differ slightly.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
A thermal interface material is any material that is inserted between two components in order to enhance the thermal coupling between them. A common use is heat dissipation, in which the TIM is inserted between a heat-producing device and a heat-dissipating device. There are intensive studies in developing several kinds of TIM with different target applications:
A hermetic seal is any type of sealing that makes a given object airtight. The term originally applied to airtight glass containers, but as technology advanced it applied to a larger category of materials, including rubber and plastics. Hermetic seals are essential to the correct and safe functionality of many electronic and healthcare products. Used technically, it is stated in conjunction with a specific test method and conditions of use.

Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste.
Thermal adhesive is a type of thermally conductive glue used for electronic components and heat sinks. It can be available as a paste or as a double-sided tape.
A diffusion barrier is a thin layer of metal usually placed between two other metals. It is done to act as a barrier to protect either one of the metals from corrupting the other.

Arctic Silver Inc. is a privately owned engineering corporation which develops and manufactures thermally conductive compounds and thermal adhesives for the application of heat sinks to high-powered electronic components such as processors, LEDs, chipsets and other electronic devices. Founded in 1999, the company's facilities are located in Visalia, California, US.

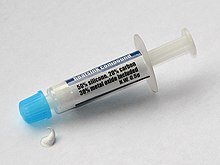
Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.

All electronic devices and circuitry generate excess heat and thus require thermal management to improve reliability and prevent premature failure. The amount of heat output is equal to the power input, if there are no other energy interactions. There are several techniques for cooling including various styles of heat sinks, thermoelectric coolers, forced air systems and fans, heat pipes, and others. In cases of extreme low environmental temperatures, it may actually be necessary to heat the electronic components to achieve satisfactory operation.

In computing and electronics, thermal pads are pre-formed rectangles of solid material commonly found on the underside of heatsinks to aid the conduction of heat away from the component being cooled and into the heatsink. Thermal pads and thermal compound are used to fill air gaps caused by imperfectly flat or smooth surfaces which should be in thermal contact; they would not be needed between perfectly flat and smooth surfaces. Thermal pads are relatively firm at room temperature, but become soft and are able to fill gaps at higher temperatures.
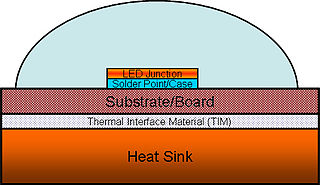
High power light-emitting diodes (LEDs) can use 350 milliwatts or more in a single LED. Most of the electricity in an LED becomes heat rather than light. If this heat is not removed, the LEDs run at high temperatures, which not only lowers their efficiency, but also makes the LED less reliable. Thus, thermal management of high power LEDs is a crucial area of research and development. It is necessary to limit both the junction and the phosphor particles temperatures to a value that will guarantee the desired LED lifetime.
A heat spreader transfers energy as heat from a hotter source to a colder heat sink or heat exchanger. There are two thermodynamic types, passive and active. The most common sort of passive heat spreader is a plate or block of material having high thermal conductivity, such as copper, aluminum, or diamond. An active heat spreader speeds up heat transfer with expenditure of energy as work supplied by an external source.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

Electronic components have a wide range of failure modes. These can be classified in various ways, such as by time or cause. Failures can be caused by excess temperature, excess current or voltage, ionizing radiation, mechanical shock, stress or impact, and many other causes. In semiconductor devices, problems in the device package may cause failures due to contamination, mechanical stress of the device, or open or short circuits.
Glass frit bonding, also referred to as glass soldering or seal glass bonding, describes a wafer bonding technique with an intermediate glass layer. It is a widely used encapsulation technology for surface micro-machined structures, e.g., accelerometers or gyroscopes. This technique utilizes low melting-point glass and therefore provides various advantages including that viscosity of glass decreases with an increase of temperature. The viscous flow of glass has effects to compensate and planarize surface irregularities, convenient for bonding wafers with a high roughness due to plasma etching or deposition. A low viscosity promotes hermetically sealed encapsulation of structures based on a better adaption of the structured shapes. Further, the coefficient of thermal expansion (CTE) of the glass material is adapted to silicon. This results in low stress in the bonded wafer pair. The glass has to flow and wet the soldered surfaces well below the temperature where deformation or degradation of either of the joined materials or nearby structures occurs. The usual temperature of achieving flowing and wetting is between 450 and 550 °C.