
Gentamicin is an antibiotic used to treat several types of bacterial infections. This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not effective for gonorrhea or chlamydia infections. It can be given intravenously, by intramuscular injection, or topically. Topical formulations may be used in burns or for infections of the outside of the eye. It is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to. The dose required should be monitored by blood testing.

Lake Hévíz is located in Hévíz, Hungary, near the western end of Lake Balaton, 8 kilometres (5 mi) from Keszthely.
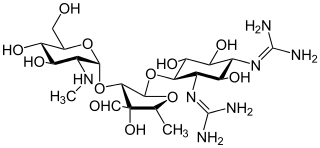
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Micromonospora is a genus of bacteria of the family Micromonosporaceae. They are gram-positive, spore-forming, generally aerobic, and form a branched mycelium; they occur as saprotrophic forms in soil and water. Various species are sources of aminoglycoside antibiotics with spellings that end with -micin, such as gentamicin, mutamicin, netilmicin, retymicin, sisomicin, verdamicin, calicheamicin, and the recently found turbinmicin. Potent new antifungal discovered in the microbiome of marine animals, unlike most other aminoglycoside names that end with -mycin.
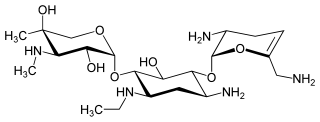
Netilmicin (1-N-ethylsisomicin) is a semisynthetic aminoglycoside antibiotic, and a derivative of sisomicin, produced by Micromonospora inyoensis. Aminoglycoside antibiotics have the ability to kill a wide variety of bacteria. Netilmicin is not absorbed from the gut and is therefore only given by injection or infusion. It is only used in the treatment of serious infections particularly those resistant to gentamicin.

The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora, with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the most potent antitumor agents known.
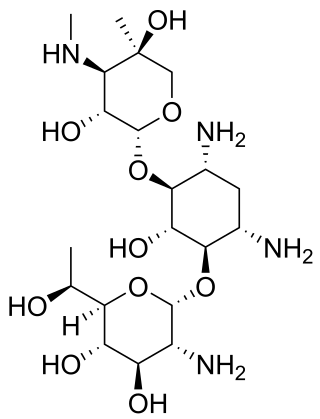
G418 (Geneticin) is an aminoglycoside antibiotic similar in structure to gentamicin B1. It is produced by Micromonospora rhodorangea. G418 blocks polypeptide synthesis by inhibiting the elongation step in both prokaryotic and eukaryotic cells. Resistance to G418 is conferred by the neo gene from Tn5 encoding an aminoglycoside 3'-phosphotransferase, APT 3' II. G418 is an analog of neomycin sulfate, and has similar mechanism as neomycin. G418 is commonly used in laboratory research to select genetically engineered cells. In general for bacteria and algae concentrations of 5 μg/mL or less are used, for mammalian cells concentrations of approximately 400 μg/mL are used for selection and 200 μg/mL for maintenance. However, optimal concentration for resistant clones selection in mammalian cells depends on the cell line used as well as on the plasmid carrying the resistance gene, therefore antibiotic titration should be done to find the best condition for every experimental system. Titration should be done using antibiotic concentrations ranging from 100 μg/mL up to 1400 μg/mL. Resistant clones selection could require from 1 to up to 3 weeks.

Desosamine is a 3-(dimethylamino)-3,4,6-trideoxyhexose found in certain macrolide antibiotics such as the commonly prescribed erythromycin, azithromycin, clarithroymcin, methymycin, narbomycin, oleandomycin, picromycin and roxithromycin. As the name suggests, these macrolide antibiotics contain a macrolide or lactone ring and they are attached to the ring Desosamine which is crucial for bactericidal activity. The biological action of the desosamine-based macrolide antibiotics is to inhibit the bacterial ribosomal protein synthesis. These antibiotics which contain Desosamine are widely used to cure bacterial-causing infections in human respiratory system, skin, muscle tissues, and urethra.

Sisomicin, is an aminoglycoside antibiotic, isolated from the fermentation broth of Micromonospora inositola. It is a newer broad-spectrum aminoglycoside most structurally related to gentamicin.
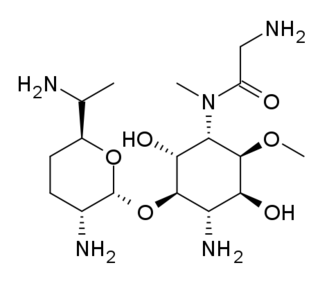
Astromicin (INN)(also frequently referenced in scientific journal articles as compounds Fortimicin A/B ) is an aminoglycoside antibiotic. Synthesized from Micromonospora olivasterospora(also named with additional o in olivoasterospora).

Micronomicin (INN) is an aminoglycoside antibiotic for use on the eye.

In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond.
Micromonospora echinospora is a species of bacteria that is known for producing the enediyne antibiotic calicheamicins.

Galtamycin B is a chemical compound that has been isolated from Micromonospora.
Micromonospora gifhornensis is a Gram-positive bacterium from the genus Micromonospora. It is Gram-positive, aerobic and spore-forming.
Micromonospora citrea is an endophytic actinomycete. It produces citreamicins, several types of antibacterial antibiotics.
Micromonospora sagamiensis is an endophytic actinomycete. It produces sagamicin, an aminoglycoside antibiotic, as well as several mutational variants. Its cell wall contains only D-alanine.

Thiocoraline is a microbial natural product of the depsipeptide class. Thiocoraline was isolated from the mycelium cake of a marine actinomycete strain L-13-ACM2-092. In vitro, thiocoraline causes an arrest in G1 phase of the cell cycle and decreases the rate of S phase progression towards G2/M phase. Thiocoraline is likely to be a DNA replication inhibitor. Thiocoraline is produced on a nonribosomal peptide synthetase (NRPS) assembly line.
Micromonospora fluostatini is a bacterium from the genus Micromonospora which has been isolated from marine sediments from Panwa Cape, Phuket Province, Thailand. Micromonospora fluostatini produces the antibiotics fluostatin B and fluostatin C
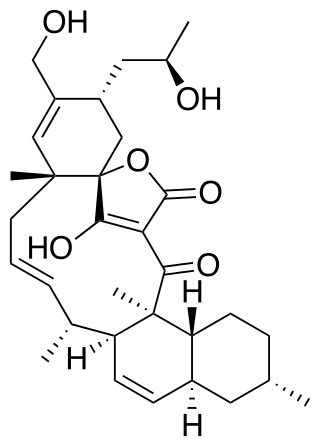
Maklamicin is a spirotetronate-class polyketide natural product. Isolated from Micromonospora sp. GMKU326 found in the root of Maklam phueak, it displays antibiotic activity against Gram-positive bacterial strains Micrococcus luteus, Bacillus subtilis, Bacillius cereus, Staphylococcus aureus, and Enterococcus faecalis.















