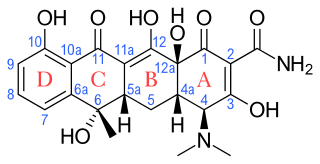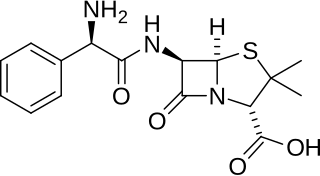
Ampicillin is an antibiotic belonging to the aminopenicillin class of the penicillin family. The drug is used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously.

Chloramphenicol is an antibiotic useful for the treatment of a number of bacterial infections. This includes use as an eye ointment to treat conjunctivitis. By mouth or by injection into a vein, it is used to treat meningitis, plague, cholera, and typhoid fever. Its use by mouth or by injection is only recommended when safer antibiotics cannot be used. Monitoring both blood levels of the medication and blood cell levels every two days is recommended during treatment.
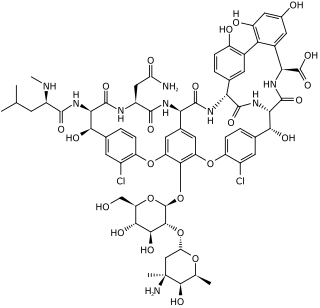
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is used intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally as a treatment for severe Clostridium difficile colitis. When taken orally it is poorly absorbed.

Pentamidine is an antimicrobial medication used to treat African trypanosomiasis, leishmaniasis, Balamuthia infections, babesiosis, and to prevent and treat pneumocystis pneumonia (PCP) in people with poor immune function. In African trypanosomiasis it is used for early disease before central nervous system involvement, as a second line option to suramin. It is an option for both visceral leishmaniasis and cutaneous leishmaniasis. Pentamidine can be given by injection into a vein or muscle or by inhalation.

Streptomycin is an antibiotic medication used to treat a number of bacterial infections, including tuberculosis, Mycobacterium avium complex, endocarditis, brucellosis, Burkholderia infection, plague, tularemia, and rat bite fever. For active tuberculosis it is often given together with isoniazid, rifampicin, and pyrazinamide. It is administered by injection into a vein or muscle.

Neomycin is an aminoglycoside antibiotic that displays bactericidal activity against Gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against Gram-positive bacilli and anaerobic Gram-negative bacilli. Neomycin comes in oral and topical formulations, including creams, ointments, and eyedrops. Neomycin belongs to the aminoglycoside class of antibiotics that contain two or more amino sugars connected by glycosidic bonds.

Gentamicin is an aminoglycoside antibiotic used to treat several types of bacterial infections. This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not effective for gonorrhea or chlamydia infections. It can be given intravenously, by intramuscular injection, or topically. Topical formulations may be used in burns or for infections of the outside of the eye. It is often only used for two days until bacterial cultures determine what specific antibiotics the infection is sensitive to. The dose required should be monitored by blood testing.
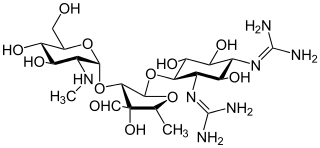
Aminoglycoside is a medicinal and bacteriologic category of traditional Gram-negative antibacterial medications that inhibit protein synthesis and contain as a portion of the molecule an amino-modified glycoside (sugar). The term can also refer more generally to any organic molecule that contains amino sugar substructures. Aminoglycoside antibiotics display bactericidal activity against Gram-negative aerobes and some anaerobic bacilli where resistance has not yet arisen but generally not against Gram-positive and anaerobic Gram-negative bacteria.

Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. For certain infections it is given with flucytosine. It is typically given intravenously.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.

Kanamycin A, often referred to simply as kanamycin, is an antibiotic used to treat severe bacterial infections and tuberculosis. It is not a first line treatment. It is used by mouth, injection into a vein, or injection into a muscle. Kanamycin is recommended for short-term use only, usually from 7 to 10 days. Since antibiotics only show activity against bacteria, it is ineffective in viral infections.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Amikacin is an antibiotic medication used for a number of bacterial infections. This includes joint infections, intra-abdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections. It is also used for the treatment of multidrug-resistant tuberculosis. It is used by injection into a vein using an IV or into a muscle.
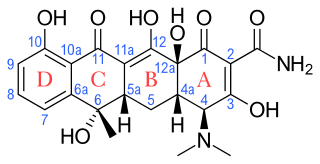
Tetracyclines are a group of broad-spectrum antibiotic compounds that have a common basic structure and are either isolated directly from several species of Streptomyces bacteria or produced semi-synthetically from those isolated compounds. Tetracycline molecules comprise a linear fused tetracyclic nucleus to which a variety of functional groups are attached. Tetracyclines are named after their four ("tetra-") hydrocarbon rings ("-cycl-") derivation ("-ine"). They are defined as a subclass of polyketides, having an octahydrotetracene-2-carboxamide skeleton and are known as derivatives of polycyclic naphthacene carboxamide. While all tetracyclines have a common structure, they differ from each other by the presence of chloro, methyl, and hydroxyl groups. These modifications do not change their broad antibacterial activity, but do affect pharmacological properties such as half-life and binding to proteins in serum.

Capreomycin is an antibiotic which is given in combination with other antibiotics for the treatment of tuberculosis. Specifically it is a second line treatment used for active drug resistant tuberculosis. It is given by injection into a vein or muscle.

Sodium stibogluconate, sold under the brand name Pentostam among others, is a medication used to treat leishmaniasis. This includes leishmaniasis of the cutaneous, visceral, and mucosal types. Some combination of miltefosine, paromomycin and liposomal amphotericin B, however, may be recommended due to issues with resistance. It is given by injection.

Spectinomycin, sold under the tradename Trobicin among others, is an antibiotic useful for the treatment of gonorrhea infections. It is given by injection into a muscle.
Antiprotozoal agents is a class of pharmaceuticals used in treatment of protozoan infection.

Arbekacin (INN) is a semisynthetic aminoglycoside antibiotic which was derived from kanamycin. It is primarily used for the treatment of infections caused by multi-resistant bacteria including methicillin-resistant Staphylococcus aureus (MRSA). Arbekacin was originally synthesized from dibekacin in 1973 by Hamao Umezawa and collaborators. It has been registered and marketed in Japan since 1990 under the trade name Habekacin. Arbekacin is no longer covered by patent and generic versions of the drug are also available under such trade names as Decontasin and Blubatosine.

Plazomicin, sold under the brand name Zemdri, is an aminoglycoside antibiotic used to treat complicated urinary tract infections. As of 2019 it is recommended only for those in whom alternatives are not an option. It is given by injection into a vein.