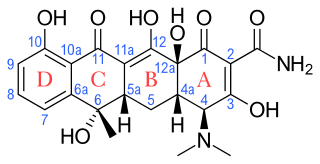Mupirocin, sold under the brand name Bactroban among others, is a topical antibiotic useful against superficial skin infections such as impetigo or folliculitis. It may also be used to get rid of methicillin-resistant S. aureus (MRSA) when present in the nose without symptoms. Due to concerns of developing resistance, use for greater than ten days is not recommended. It is used as a cream or ointment applied to the skin.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.
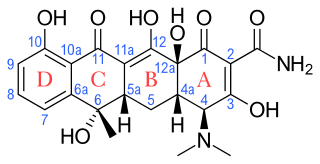
Tetracyclines are a group of broad-spectrum antibiotic compounds that have a common basic structure and are either isolated directly from several species of Streptomyces bacteria or produced semi-synthetically from those isolated compounds. Tetracycline molecules comprise a linear fused tetracyclic nucleus to which a variety of functional groups are attached. Tetracyclines are named after their four ("tetra-") hydrocarbon rings ("-cycl-") derivation ("-ine"). They are defined as a subclass of polyketides, having an octahydrotetracene-2-carboxamide skeleton and are known as derivatives of polycyclic naphthacene carboxamide. While all tetracyclines have a common structure, they differ from each other by the presence of chloro, methyl, and hydroxyl groups. These modifications do not change their broad antibacterial activity, but do affect pharmacological properties such as half-life and binding to proteins in serum.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Oleandomycin is a macrolide antibiotic. It is synthesized from strains of Streptomyces antibioticus. It is weaker than erythromycin.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.

Monocerin is a dihydroisocoumarin and a polyketide metabolite that originates from various fungal species. It has been shown to display antifungal, plant pathogenic, and insecticidal characteristics. Monocerin has been isolated from Dreschlera monoceras, D. ravenelii, Exserohilum turcicum, and Fusarium larvarum.

In molecular biology, the polyketide synthesis cyclase family of proteins includes a number of cyclases involved in polyketide synthesis in a number of actinobacterial species.
Streptomyces rimosus is a bacterium species in the genus Streptomyces.

Anthracimycin is a polyketide antibiotic discovered in 2013. Anthracimycin is derived from marine actinobacteria. In preliminary laboratory research, it has shown activity against Bacillus anthracis, the bacteria that causes anthrax, and against methicillin-resistant Staphylococcus aureus (MRSA).
Marinone is an antibiotic made by marine actinomycetes.
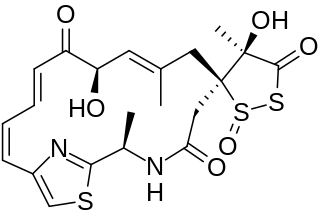
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Butyrolactol A is an organic chemical compound of interest for its potential use as an antifungal antibiotic.
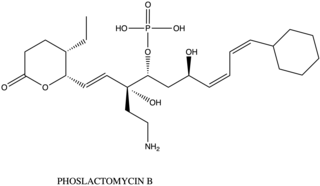
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.
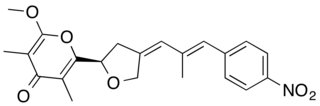
Aureothin is a natural product of a cytotoxic shikimate-polyketide antibiotic with the molecular formula C22H23NO6. Aureothin is produced by the bacterium Streptomyces thioluteus that illustrates antitumor, antifungal, and insecticidal activities and the new aureothin derivatives can be antifungal and antiproliferative. In addition, aureothin, a nitro compound from Streptomyces thioluteus, was indicated to have pesticidal activity against the bean weevil by interfering with mitochondrial respiratory complex II.

Tetracenomycin C is an antitumor anthracycline-like antibiotic produced by Streptomyces glaucescens GLA.0. The pale-yellow antibiotic is active against some gram-positive bacteria, especially against streptomycetes. Gram-negative bacteria and fungi are not inhibited. In considering the differences of biological activity and the functional groups of the molecule, tetracenomycin C is not a member of the tetracycline or anthracyclinone group of antibiotics. Tetracenomycin C is notable for its broad activity against actinomycetes. As in other anthracycline antibiotics, the framework is synthesized by a polyketide synthase and subsequently modified by other enzymes.

Prescopranone is a key intermediate in the biosynthesis of scopranones. Prescopranone is the precursor to scopranone A, scopranone B, and scopranone C, which are produced by Streptomyces sp. BYK-11038.