
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as peripheral blood, and the blood cells it carries, peripheral blood cells.

Oncotic pressure, or colloid osmotic-pressure, is a type of osmotic pressure induced by the plasma proteins, notably albumin, in a blood vessel's plasma that causes a pull on fluid back into the capillary. Participating colloids displace water molecules, thus creating a relative water molecule deficit with water molecules moving back into the circulatory system within the lower venous pressure end of capillaries.

Shock is the state of insufficient blood flow to the tissues of the body as a result of problems with the circulatory system. Initial symptoms of shock may include weakness, fast heart rate, fast breathing, sweating, anxiety, and increased thirst. This may be followed by confusion, unconsciousness, or cardiac arrest, as complications worsen.

Intravenous therapy is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use.

Fluid replacement or fluid resuscitation is the medical practice of replenishing bodily fluid lost through sweating, bleeding, fluid shifts or other pathologic processes. Fluids can be replaced with oral rehydration therapy (drinking), intravenous therapy, rectally such as with a Murphy drip, or by hypodermoclysis, the direct injection of fluid into the subcutaneous tissue. Fluids administered by the oral and hypodermic routes are absorbed more slowly than those given intravenously.

Hypovolemic shock is a form of shock caused by severe hypovolemia. It could be the result of severe dehydration through a variety of mechanisms or blood loss. Hypovolemic shock is a medical emergency; if left untreated, the insufficient blood flow can cause damage to organs, leading to multiple organ failure.
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood. Early symptoms may include a strong feeling of thirst, weakness, nausea, and loss of appetite. Severe symptoms include confusion, muscle twitching, and bleeding in or around the brain. Normal serum sodium levels are 135–145 mmol/L. Hypernatremia is generally defined as a serum sodium level of more than 145 mmol/L. Severe symptoms typically only occur when levels are above 160 mmol/L.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer's, and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.
A post-anesthesia care unit, often abbreviated PACU and sometimes referred to as post-anesthesia recovery or PAR, or simply recovery, is a part of hospitals, ambulatory care centers, and other medical facilities. Patients who received general anesthesia, regional anesthesia, or local anesthesia are transferred from the operating room suites to the recovery area. The patients are monitored typically by anesthesiologists, nurse anesthetists, and other medical staff. Providers follow a standardized handoff to the medical PACU staff that includes, which medications were given in the operating room suites, how hemodynamics were during the procedures, and what is expected for their recovery. After initial assessment and stabilization, patients are monitored for any potential complications, until the patient is transferred back to their hospital rooms.

Hydroxyethyl starch (HES/HAES), sold under the brand name Voluven among others, is a nonionic starch derivative, used as a volume expander in intravenous therapy. The use of HES on critically ill patients is associated with an increased risk of death and kidney problems.
Early goal-directed therapy was introduced by Emanuel P. Rivers in The New England Journal of Medicine in 2001 and is a technique used in critical care medicine involving intensive monitoring and aggressive management of perioperative hemodynamics in patients with a high risk of morbidity and mortality. In cardiac surgery, goal-directed therapy has proved effective when commenced after surgery. The combination of GDT and Point-of-Care Testing has demonstrated a marked decrease in mortality for patients undergoing congenital heart surgery. Furthermore, a reduction in morbidity and mortality has been associated with GDT techniques when used in conjunction with an electronic medical record.
Pentastarch is a subgroup of hydroxyethyl starch, with five hydroxyethyl groups out of each 11 hydroxyls, giving it approximately 50% hydroxyethylation. This compares with tetrastarch at 40% and hetastarch at 70% hydroxyethylation, respectively.

Ringer's solution is a solution of several salts dissolved in water for the purpose of creating an isotonic solution relative to the body fluids of an animal. Ringer's solution typically contains sodium chloride, potassium chloride, calcium chloride and sodium bicarbonate, with the last used to balance the pH. Other additions can include chemical fuel sources for cells, including ATP and dextrose, as well as antibiotics and antifungals.
An antihypotensive agent, also known as a vasopressor agent or simply vasopressor, or pressor, is any substance, whether endogenous or a medication, that tends to raise low blood pressure. Some antihypotensive drugs act as vasoconstrictors to increase total peripheral resistance; some drugs sensitize adrenoreceptors to catecholamines; and others increase cardiac output.
A balanced salt solution (BSS) is a solution made to a physiological pH and isotonic salt concentration. Solutions most commonly include sodium, potassium, calcium, magnesium, and chloride. Balanced salt solutions are used for washing tissues and cells and are usually combined with other agents to treat the tissues and cells. They provide the cells with water and inorganic ions, while maintaining a physiological pH and osmotic pressure.
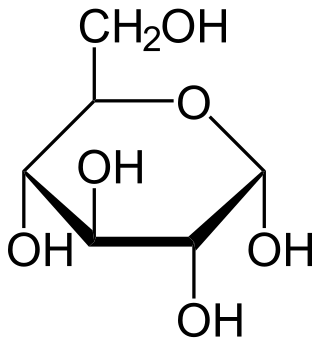
Intravenous sugar solution, also known as dextrose solution, is a mixture of dextrose (glucose) and water. It is used to treat low blood sugar or water loss without electrolyte loss. Water loss without electrolyte loss may occur in fever, hyperthyroidism, high blood calcium, or diabetes insipidus. It is also used in the treatment of high blood potassium, diabetic ketoacidosis, and as part of parenteral nutrition. It is given by injection into a vein.
Permissive hypotension or hypotensive resuscitation is the use of restrictive fluid therapy, specifically in the trauma patient, that increases systemic blood pressure without reaching normotension. The goal blood pressure for these patients is a mean arterial pressure of 40-50 mmHg or systolic blood pressure of less than or equal to 80. This goes along with certain clinical criteria. Following traumatic injury, some patients experience hypotension that is usually due to blood loss (hemorrhage) but can be due to other causes as well. In the past, physicians were very aggressive with fluid resuscitation to try to bring the blood pressure to normal values. Recent studies have found that there is some benefit to allowing specific patients to experience some degree of hypotension in certain settings. This concept does not exclude therapy by means of i.v. fluid, inotropes or vasopressors, the only restriction is to avoid completely normalizing blood pressure in a context where blood loss may be enhanced. When a person starts to bleed the body starts a natural coagulation process that eventually stops the bleed. Issues with fluid resuscitation without control of bleeding are thought to be secondary to dislodgement of the thrombus that is helping to control further bleeding. Thrombus dislodgement was found to occur at a systolic pressure greater than 80mm Hg. In addition, fluid resuscitation will dilute coagulation factors that help form and stabilize a clot, hence making it harder for the body to use its natural mechanisms to stop the bleeding. These factors are aggravated by hypothermia.
Pedro J. del Nido is an American pediatric cardiac surgeon who was the 95th president of the American Association for Thoracic Surgery (AATS), succeeding David J. Sugarbaker and preceding Joseph S. Coselli.










