Zirconium is a chemical element; it has symbol Zr and atomic number 40. The name zirconium is derived from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian zargun. It is a lustrous, grey-white, strong transition metal that closely resembles hafnium and, to a lesser extent, titanium. Zirconium is mainly used as a refractory and opacifier, although small amounts are used as an alloying agent for its strong resistance to corrosion. Zirconium forms a variety of inorganic and organometallic compounds such as zirconium dioxide and zirconocene dichloride, respectively. Five isotopes occur naturally, four of which are stable. Zirconium compounds have no known biological role.
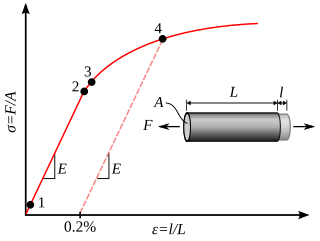
In physics and materials science, plasticity is the ability of a solid material to undergo permanent deformation, a non-reversible change of shape in response to applied forces. For example, a solid piece of metal being bent or pounded into a new shape displays plasticity as permanent changes occur within the material itself. In engineering, the transition from elastic behavior to plastic behavior is known as yielding.

A loss-of-coolant accident (LOCA) is a mode of failure for a nuclear reactor; if not managed effectively, the results of a LOCA could result in reactor core damage. Each nuclear plant's emergency core cooling system (ECCS) exists specifically to deal with a LOCA.

Crystal twinning occurs when two or more adjacent crystals of the same mineral are oriented so that they share some of the same crystal lattice points in a symmetrical manner. The result is an intergrowth of two separate crystals that are tightly bonded to each other. The surface along which the lattice points are shared in twinned crystals is called a composition surface or twin plane.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.
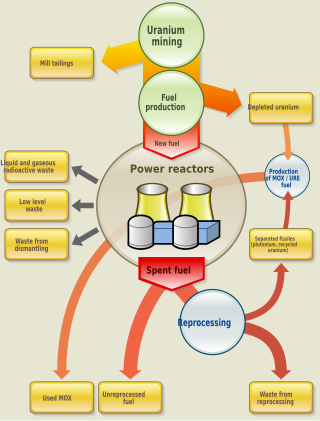
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.

Lüders bands, is type of slip bands in metals or stretcher-strain marks which are formed due to localized bands of plastic deformation in metals experiencing tensile stresses, common to low-carbon steels and certain Al-Mg alloys. First reported by Guillaume Piobert, and later by W. Lüders, the mechanism that stimulates their appearance is known as dynamic strain aging, or the inhibition of dislocation motion by interstitial atoms, around which "atmospheres" or "zones" naturally congregate.
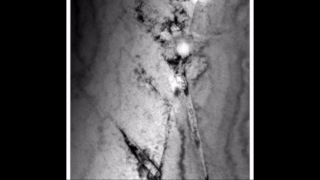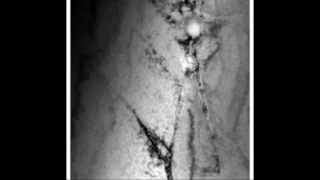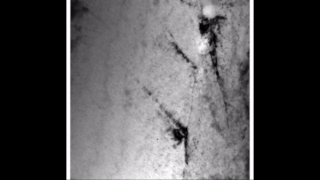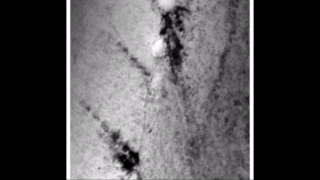
Zirconium hydride describes an alloy made by combining zirconium and hydrogen. Hydrogen acts as a hardening agent, preventing dislocations in the zirconium atom crystal lattice from sliding past one another. Varying the amount of hydrogen and the form of its presence in the zirconium hydride controls qualities such as the hardness, ductility, and tensile strength of the resulting zirconium hydride. Zirconium hydride with increased hydrogen content can be made harder and stronger than zirconium, but such zirconium hydride is also less ductile than zirconium.

In materials science, slip is the large displacement of one part of a crystal relative to another part along crystallographic planes and directions. Slip occurs by the passage of dislocations on close/packed planes, which are planes containing the greatest number of atoms per area and in close-packed directions. Close-packed planes are known as slip or glide planes. A slip system describes the set of symmetrically identical slip planes and associated family of slip directions for which dislocation motion can easily occur and lead to plastic deformation. The magnitude and direction of slip are represented by the Burgers vector, b.
Magnesium alloys are mixtures of magnesium with other metals, often aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Magnesium alloys have a hexagonal lattice structure, which affects the fundamental properties of these alloys. Plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel; therefore, magnesium alloys are typically used as cast alloys, but research of wrought alloys has been more extensive since 2003. Cast magnesium alloys are used for many components of modern automobiles and have been used in some high-performance vehicles; die-cast magnesium is also used for camera bodies and components in lenses.
This page describes how uranium dioxide nuclear fuel behaves during both normal nuclear reactor operation and under reactor accident conditions, such as overheating. Work in this area is often very expensive to conduct, and so has often been performed on a collaborative basis between groups of countries, usually under the aegis of the Organisation for Economic Co-operation and Development's Committee on the Safety of Nuclear Installations (CSNI).

A shear band is a narrow zone of intense shearing strain, usually of plastic nature, developing during severe deformation of ductile materials. As an example, a soil specimen is shown in Fig. 1, after an axialsymmetric compression test. Initially the sample was cylindrical in shape and, since symmetry was tried to be preserved during the test, the cylindrical shape was maintained for a while during the test and the deformation was homogeneous, but at extreme loading two X-shaped shear bands had formed and the subsequent deformation was strongly localized.
Oxide dispersion strengthened alloys (ODS) are alloys that consist of a metal matrix with small oxide particles dispersed within it. They have high heat resistance, strength, and ductility. Alloys of nickel are the most common but includes iron aluminum alloys.
Severe plastic deformation (SPD) is a generic term describing a group of metalworking techniques involving very large strains typically involving a complex stress state or high shear, resulting in a high defect density and equiaxed "ultrafine" grain (UFG) size or nanocrystalline (NC) structure.
In materials science, the yield strength anomaly refers to materials wherein the yield strength increases with temperature. For the majority of materials, the yield strength decreases with increasing temperature. In metals, this decrease in yield strength is due to the thermal activation of dislocation motion, resulting in easier plastic deformation at higher temperatures.

Corium, also called fuel-containing material (FCM) or lava-like fuel-containing material (LFCM), is a material that is created in a nuclear reactor core during a nuclear meltdown accident. Resembling lava in consistency, it consists of a mixture of nuclear fuel, fission products, control rods, structural materials from the affected parts of the reactor, products of their chemical reaction with air, water, steam, and in the event that the reactor vessel is breached, molten concrete from the floor of the reactor room.
Ultra-high-temperature ceramics (UHTCs) are a type of refractory ceramics that can withstand extremely high temperatures without degrading, often above 2,000 °C. They also often have high thermal conductivities and are highly resistant to thermal shock, meaning they can withstand sudden and extreme changes in temperature without cracking or breaking. Chemically, they are usually borides, carbides, nitrides, and oxides of early transition metals.
Friction extrusion is a thermo-mechanical process that can be used to form fully consolidated wire, rods, tubes, or other non-circular metal shapes directly from a variety of precursor charges including metal powder, flake, machining waste or solid billet. The process imparts unique, and potentially, highly desirable microstructures to the resulting products. Friction extrusion was invented at The Welding Institute in the UK and patented in 1991. It was originally intended primarily as a method for production of homogeneous microstructures and particle distributions in metal matrix composite materials.
Crystal plasticity is a mesoscale computational technique that takes into account crystallographic anisotropy in modelling the mechanical behaviour of polycrystalline materials. The technique has typically been used to study deformation through the process of slip, however, there are some flavors of crystal plasticity that can incorporate other deformation mechanisms like twinning and phase transformations. Crystal plasticity is used to obtain the relationship between stress and strain that also captures the underlying physics at the crystal level. Hence, it can be used to predict not just the stress-strain response of a material, but also the texture evolution, micromechanical field distributions, and regions of strain localisation. The two widely used formulations of crystal plasticity are the one based on the finite element method known as Crystal Plasticity Finite Element Method (CPFEM), which is developed based on the finite strain formulation for the mechanics, and a spectral formulation which is more computationally efficient due to the fast Fourier transform, but is based on the small strain formulation for the mechanics.
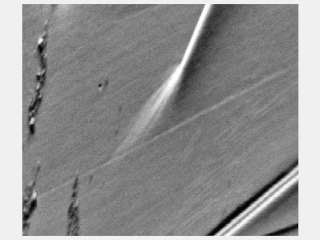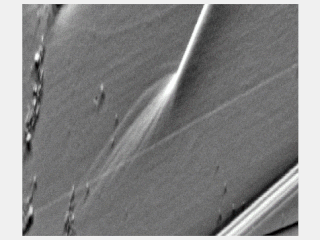
Slip bands or stretcher-strain marks are localized bands of plastic deformation in metals experiencing stresses. Formation of slip bands indicates a concentrated unidirectional slip on certain planes causing a stress concentration. Typically, slip bands induce surface steps and a stress concentration which can be a crack nucleation site. Slip bands extend until impinged by a boundary, and the generated stress from dislocations pile-up against that boundary will either stop or transmit the operating slip depending on its (mis)orientation.