Related Research Articles

Allantoin is a chemical compound with formula C4H6N4O3. It is also called 5-ureidohydantoin or glyoxyldiureide. It is a diureide of glyoxylic acid. Allantoin is a major metabolic intermediate in most organisms including animals, plants and bacteria. It is produced from uric acid, which itself is a degradation product of nucleic acids, by action of urate oxidase (uricase). The occurs as a natural mineral compound (IMA symbol Aan).

The enzyme urate oxidase (UO), uricase or factor-independent urate hydroxylase, absent in humans, catalyzes the oxidation of uric acid to 5-hydroxyisourate:

Orotidine 5'-phosphate decarboxylase or orotidylate decarboxylase is an enzyme involved in pyrimidine biosynthesis. It catalyzes the decarboxylation of orotidine monophosphate (OMP) to form uridine monophosphate (UMP). The function of this enzyme is essential to the de novo biosynthesis of the pyrimidine nucleotides uridine triphosphate, cytidine triphosphate, and thymidine triphosphate. OMP decarboxylase has been a frequent target for scientific investigation because of its demonstrated extreme catalytic efficiency and its usefulness as a selection marker for yeast strain engineering.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.
The crotonase family comprises mechanistically diverse proteins that share a conserved trimeric quaternary structure, the core of which consists of 4 turns of a (beta/beta/alpha)n superhelix.

In enzymology, a homoserine dehydrogenase (EC 1.1.1.3) is an enzyme that catalyzes the chemical reaction
The enzyme acetolactate decarboxylase (EC 4.1.1.5) catalyzes the chemical reaction
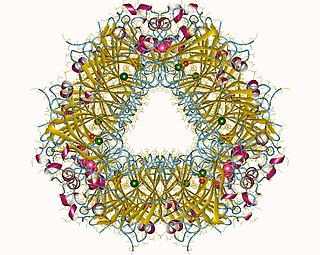
In enzymology, an oxalate decarboxylase (EC 4.1.1.2) is an oxalate degrading enzyme that catalyzes the chemical reaction
The enzyme threonine-phosphate decarboxylase (EC 4.1.1.81) catalyzes the chemical reaction

In molecular biology, the protein domain SAICAR synthase is an enzyme which catalyses a reaction to create SAICAR. In enzymology, this enzyme is also known as phosphoribosylaminoimidazolesuccinocarboxamide synthase. It is an enzyme that catalyzes the chemical reaction
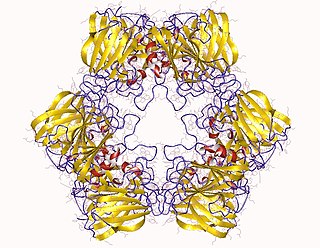
Allantoicase is an enzyme (EC 3.5.3.4) that in humans is encoded by the ALLC gene. Allantoicase catalyzes the chemical reaction
In enzymology, a hydroxyisourate hydrolase (EC 3.5.2.17) is an enzyme that catalyzes the chemical reaction

Oxoeicosanoid receptor 1 (OXER1) also known as G-protein coupled receptor 170 (GPR170) is a protein that in humans is encoded by the OXER1 gene located on human chromosome 2p21; it is the principal receptor for the 5-Hydroxyicosatetraenoic acid family of carboxy fatty acid metabolites derived from arachidonic acid. The receptor has also been termed hGPCR48, HGPCR48, and R527 but OXER1 is now its preferred designation. OXER1 is a G protein-coupled receptor (GPCR) that is structurally related to the hydroxy-carboxylic acid (HCA) family of G protein-coupled receptors whose three members are HCA1 (GPR81), HCA2, and HCA3 ; OXER1 has 30.3%, 30.7%, and 30.7% amino acid sequence identity with these GPCRs, respectively. It is also related to the recently defined receptor, GPR31, for the hydroxyl-carboxy fatty acid 12-HETE.
In enzymology, a [3-methyl-2-oxobutanoate dehydrogenase (acetyl-transferring)] is an enzyme that catalyzes the chemical reaction

Retinoblastoma-binding protein 8 is a protein that in humans is encoded by the RBBP8 gene.

Ornithine decarboxylase antizyme is an enzyme that in humans is encoded by the OAZ1 gene.
A ureohydrolase is a type of hydrolase enzyme.

The FPG IleRS zinc finger domain represents a zinc finger domain found at the C-terminal in both DNA glycosylase/AP lyase enzymes and in isoleucyl tRNA synthetase. In these two types of enzymes, the C-terminal domain forms a zinc finger.

Cyanophycinase (EC 3.4.15.6, cyanophycin degrading enzyme, beta-Asp-Arg hydrolysing enzyme, CGPase, CphB, CphE, cyanophycin granule polypeptidase, extracellular CGPase) is an enzyme. It catalyses the following chemical reaction
Carboxynorspermidine decarboxylase (EC 4.1.1.96, carboxyspermidine decarboxylase, CANSDC, VC1623 (gene)) is an enzyme with systematic name carboxynorspermidine carboxy-lyase (bis(3-aminopropyl)amine-forming). This enzyme catalyses the following chemical reaction
References
- ↑ Ramazzina I, Folli C, Secchi A, Berni R, Percudani R (March 2006). "Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes". Nat. Chem. Biol. 2 (3): 144–8. doi:10.1038/nchembio768. PMID 16462750. S2CID 13441301.
- ↑ Cendron L, Berni R, Folli C, Ramazzina I, Percudani R, Zanotti G (2007). "The structure of 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline decarboxylase provides insights into the mechanism of uric acid degradation". J Biol Chem. 282 (25): 18182–9. doi: 10.1074/jbc.M701297200 . PMID 17428786.
- ↑ Kim K, Park J, Rhee S (2007). "Structural and functional basis for (S)-allantoin formation in the ureide pathway". J Biol Chem. 282 (32): 23457–64. doi: 10.1074/jbc.M703211200 . PMID 17567580.
- ↑ Todd CD, Tipton PA, Blevins DG, Piedras P, Pineda M, Polacco JC (2006). "Update on ureide degradation in legumes". J Exp Bot. 57 (1): 5–12. doi: 10.1093/jxb/erj013 . PMID 16317038.