
A biogenic substance is a product made by or of life forms. While the term originally was specific to metabolite compounds that had toxic effects on other organisms, it has developed to encompass any constituents, secretions, and metabolites of plants or animals. In context of molecular biology, biogenic substances are referred to as biomolecules. They are generally isolated and measured through the use of chromatography and mass spectrometry techniques. Additionally, the transformation and exchange of biogenic substances can by modelled in the environment, particularly their transport in waterways.
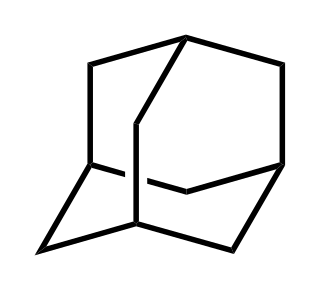
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

2-Imidazoline (Preferred IUPAC name: 4,5-dihydro-1H-imidazole) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C3H6N2. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.

Marine pharmacognosy is the investigation and identification of medically important plants and animals in the marine environment. It is a sub branch of terrestrial pharmacognosy. Generally the drugs are obtained from the marine species of bacteria, virus, algae, fungi and sponges. It is a relatively new field of study in western medicine, although many marine organisms were used in Traditional Chinese Medicine. It was not until 2004 that the first FDA approval of a drug came directly from the sea: ziconotide, which was isolated from a marine cone snail.
Organobromine compounds, also called organobromides, are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.
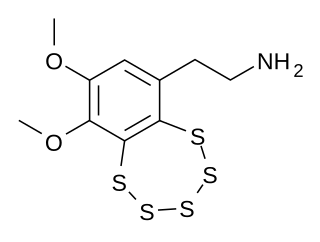
Varacin is a bicyclic organosulfur compound originally found in marine Ascidiacea from the Polycitor genus. It contains an unusual pentathiepin ring which reacts with DNA, and varacin and synthetic analogues have been investigated for their antimicrobial and antitumour properties. Because of its potent biological activity and unusual and challenging ring system, it has been a popular target of efforts toward its total synthesis.
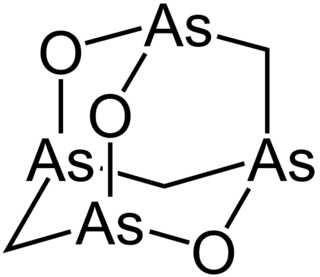
Arsenicin A is a naturally occurring organoarsenic compound with molecular formula C3H6As4O3. It was first isolated from the New Caledonian marine sponge Echinochalina bargibanti. The compound was characterized by computational and spectroscopic techniques and found to possess a cage-like structure similar to adamantane in which the four methanetriyl carbon bridgeheads are replaced by arsenic atoms and three of the six methylene bridges are replaced by oxygen atoms. It is the first polyarsenic compound ever found in nature. Subsequently, the proposed structure was prepared in large quantities via total synthesis and the structure was confirmed by x-ray crystallography. The molecule is chiral, and has been resolved into its two enantiomers. Arsenicin A is active against promelocytic leukemia cells at lower concentrations than the arsenic(III) oxide drug Trisenox.

Palau'amine is a toxic alkaloid compound synthesized naturally by Stylotella agminata, a species of sea sponge found in the southwest Pacific Ocean. The name of the molecule derives from the island nation of Palau, near which the sponges are found.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.
Tubulin inhibitors are chemotherapy drugs that interfere directly with the tubulin system, which is in contrast to those chemotherapy drugs acting on DNA. Microtubules play an important role in eukaryotic cells. Alpha- and beta-tubulin, the main components of microtubules, have gained considerable interest because of their function and biophysical properties and has become the subject of intense study. The addition of tubulin ligands can affect microtubule stability and function, including mitosis, cell motion and intracellular organelle transport. Tubulin binding molecules have generated significant interest after the introduction of the taxanes into clinical oncology and the general use of the vinca alkaloids. These compounds inhibit cell mitosis by binding to the protein tubulin in the mitotic spindle and preventing polymerization or depolymerization into the microtubules. This mode of action is also shared with another natural agent called colchicine.
Ichthyotoxins are compounds which are either toxic to fish, or are toxins produced by fish. The former include the algae-produced euglenophycin and prymnesins, which can cause large-scale fish deaths. The latter includes ostracitoxin, produced by boxfish. Many toxin-producing algal species can be found both in marine and fresh water environments when the algae are in bloom. Ichthyotoxic poisoning in humans can cause symptoms ranging in severity dependent on how much toxin was consumed. The symptoms of an ichthyotoxin poisoning from fish venoms can include headache, vomiting, diarrhea, dizziness, and drop in blood pressure.

Lacking an immune system, protective shell, or mobility, sponges have developed an ability to synthesize a variety of unusual compounds for survival. C-nucleosides isolated from Caribbean Cryptotethya crypta, were the basis for the synthesis of zidovudine (AZT), aciclovir (Cyclovir), cytarabine (Depocyt), and cytarabine derivative gemcitabine (Gemzar).
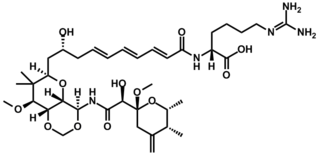
Onnamide A is a bioactive natural product found in Theonella swinhoei, a species of marine sponge whose genus is well known for yielding a diverse set of biologically active natural products, including the swinholides and polytheonamides. It bears structural similarities to the pederins, a family of compounds known to inhibit protein synthesis in eukaryotic cells. Onnamide A and its analogues have attracted academic interest due to their cytotoxicity and potential for combating the growth and proliferation of cancer cells.

Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.
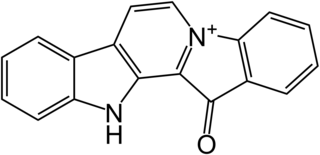
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis bergquist collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.
Latrunculia biformis, the mud-clump sponge, is a widespread deep sea demosponge from the southern hemisphere.

Avarol is a hydroquinone first isolated from the Mediterranean marine sponge Dysidea avara 1974 Avarol represented the first sesquiterpenoid with a rearranged drimane skeleton and its structure was established by standard analytical methods, chemical degradation and later by stereocontrolled synthesis. Intrigued by the wide range of biological activities of this metabolite, Avarol has inspired the development of many synthetic derivatives and the study of their applications.

Pseudoceratina is a genus of sponge within the family Pseudoceratinidae. They are characterized by possession of a dendritic fiber skeleton lacking laminar bark but containing pith. They have been found in a variety of habitats including the Great Barrier reef, the Red Sea, and Jamaica. Sponges of this genus have a microbiome known to produce a variety of chemicals that are used in pharmaceutical and anti-fouling activities. Notably, a species in this genus produces a chemical that is effective in inhibiting the migration of metastatic breast cancer cells.
















