
The Apicomplexa are a large phylum of parasitic alveolates. Most of them possess a unique form of organelle that comprises a type of plastid called an apicoplast, and an apical complex structure. The organelle is an adaptation that the apicomplexan applies in penetration of a host cell.

Plasmodium vivax is a protozoal parasite and a human pathogen. This parasite is the most frequent and widely distributed cause of recurring malaria. Although it is less virulent than Plasmodium falciparum, the deadliest of the five human malaria parasites, P. vivax malaria infections can lead to severe disease and death, often due to splenomegaly. P. vivax is carried by the female Anopheles mosquito; the males do not bite.
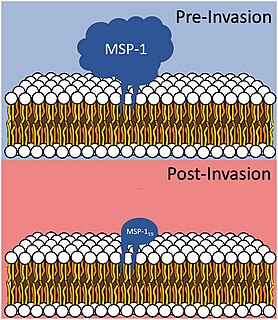
Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.

Micronemes are secretory organelles, possessed by parasitic apicomplexans. Micronemes are located on the apical third of the protozoan body. They are surrounded by a typical unit membrane. On electron microscopy they have an electron-dense matrix due to the high protein content. They are specialized secretory organelles important for host-cell invasion and gliding motility.
Subtelomeres are segments of DNA between telomeric caps and chromatin.

Plasmodium berghei is a species in the genus Plasmodium subgenus Vinckeia.
An apicoplast is a derived non-photosynthetic plastid found in most Apicomplexa, including Toxoplasma gondii, Plasmodium falciparum and other Plasmodium spp., but not in others such as Cryptosporidium. It originated from an alga through secondary endosymbiosis. The apicoplast is surrounded by four membranes within the outermost part of the endomembrane system. The apicoplast hosts important metabolic pathways like fatty acid synthesis, isoprenoid precursor synthesis and parts of the heme biosynthetic pathway
Plasmodium molecular tools are a set of methods for the genetic manipulation of the parasite genus Plasmodium. Plasmodium species have been difficult to scientifically study, partially due to the inability of many standard biological techniques to genetically alter the organism. Recent research has sought to overcome these technical barriers in order to make the parasite more amenable to study. Below is a description of published methods of genetic control within the Plasmodium parasite.
Chromera velia, also known as a "chromerid", is a unicellular photosynthetic organism in the superphylum Alveolata. It is of interest in the study of apicomplexan parasites, specifically their evolution and accordingly, their unique vulnerabilities to drugs.
Pregnancy-associated malaria (PAM) or placental malaria is a presentation of the common illness that is particularly life-threatening to both mother and developing fetus. PAM is caused primarily by infection with Plasmodium falciparum, the most dangerous of the four species of malaria-causing parasites that infect humans. During pregnancy, a woman faces a much higher risk of contracting malaria and of associated complications. Prevention and treatment of malaria are essential components of prenatal care in areas where the parasite is endemic.

In molecular biology, apical membrane antigen 1 is a novel antigen of Plasmodium falciparum which has been cloned. It contains a hydrophobic domain typical of an integral membrane protein. The antigen is designated apical membrane antigen 1 (AMA-1) by virtue of appearing to be located in the apical complex. AMA-1 appears to be transported to the merozoite surface close to the time of schizont rupture.
The extended EGL-27 and MTA1 homology domain, or EELM2 domain, is a protein domain that occurs in proteins from apicomplexan parasites.
The partial cleavage stimulation factor domain, or partial CstF domain, is a protein domain that occurs in proteins from apicomplexan parasites.
Circumsporozoite protein (CSP) is a secreted protein of the sporozoite stage of the malaria parasite and is the antigenic target of RTS,S, a pre-erythrocytic malaria vaccine currently undergoing clinical trials. The amino-acid sequence of CSP consists of an immunodominant central repeat region flanked by conserved motifs at the N- and C- termini that are implicated in protein processing as the parasite travels from the mosquito to the mammalian vector.
The parasitophorous vacuole (PV) is a structure produced by apicomplexan parasites in the cells of its host. The PV allows the parasite to develop while protected from the phagolysosomes of the host cell.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, rendering evasion from the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.

The Plasmodium helical interspersed subtelomeric proteins (PHIST) or ring-infected erythrocyte surface antigens (RESA) are a family of protein domains found in the malaria-causing Plasmodium species. It was initially identified as a short four-helical conserved region in the single-domain export proteins, but the identification of this part associated with a DnaJ domain in P. falciparum RESA has led to its reclassification as the RESA N-terminal domain. This domain has been classified into three subfamilies, PHISTa, PHISTb, and PHISTc.









