Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originated from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions. It relies on high pressure pumps, which deliver mixtures of various solvents, called the mobile phase, collecting the sample mixture on the way, through a column filled with a solid adsorbent material, called the stationary phase. Each component in the sample interacts slightly differently with the adsorbent material, causing different migration rates for the different components, leading to their separation, as they flow out of the column into a specific detector.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Supercritical fluid extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination) or collect a desired product (e.g. essential oils). These essential oils can include limonene and other straight solvents. Carbon dioxide (CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical carbon dioxide are above the critical temperature of 31 °C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.

Solid-phase extraction (SPE) is a solid-liquid extractive technique by which compounds that are dissolved or suspended in a liquid mixture are separated from other compounds in the mixture according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.

An extract (essence) is a substance made by extracting a part of a raw material, often by using a solvent such as ethanol, oil or water. Extracts may be sold as tinctures, absolutes or in powder form.
Solid phase microextraction, or SPME, is a solid phase extraction sampling technique that involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent), which extracts different kinds of analytes from different kinds of media, that can be in liquid or gas phase. The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds. It is typically performed during the work-up step following a chemical synthesis to purify crude compounds and results in the product being largely free of acidic or basic impurities. A separatory funnel is commonly used to perform an acid-base extraction.

Desorption electrospray ionization (DESI) is an ambient ionization technique that can be coupled to mass spectrometry (MS) for chemical analysis of samples at atmospheric conditions. Coupled ionization sources-MS systems are popular in chemical analysis because the individual capabilities of various sources combined with different MS systems allow for chemical determinations of samples. DESI employs a fast-moving charged solvent stream, at an angle relative to the sample surface, to extract analytes from the surfaces and propel the secondary ions toward the mass analyzer. This tandem technique can be used to analyze forensics analyses, pharmaceuticals, plant tissues, fruits, intact biological tissues, enzyme-substrate complexes, metabolites and polymers. Therefore, DESI-MS may be applied in a wide variety of sectors including food and drug administration, pharmaceuticals, environmental monitoring, and biotechnology.
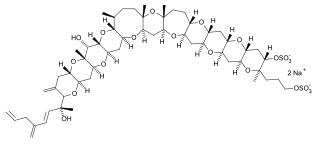
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.

Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term washing may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired compound.

Nimbin is a triterpenoid isolated from Neem. Nimbin is thought to be responsible for much of the biological activities of neem oil, and is reported to have anti-inflammatory, antipyretic, fungicidal, antihistamine and antiseptic properties.
Single cell oil, also known as Microbial oil consists of the intracellular storage lipids, triacyglycerols. It is similar to vegetable oil, another biologically produced oil. They are produced by oleaginous microorganisms, which is the term for those bacteria, molds, algae and yeast, which can accumulate 20% to 80% lipids of their biomass. The accumulation of lipids take place by the end of logarithmic phase and continues during station phase until carbon source begins to reduce with nutrition limitation.
Concrete, in perfumery, is a waxy mass obtained by solvent extraction of fresh plant material. It is usually used for the production of absolutes.
Mass spectrometric immunoassay (MSIA) is a rapid method is used to detect and/ or quantify antigens and or antibody analytes. This method uses an analyte affinity isolation to extract targeted molecules and internal standards from biological fluid in preparation for matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS). This method allows for "top down" and "bottom up" analysis. This sensitive method allows for a new and improved process for detecting multiple antigens and antibodies in a single assay. This assay is also capable of distinguishing mass shifted forms of the same molecule via a panantibody, as well as distinguish point mutations in proteins. Each specific form is detected uniquely based on their characteristic molecular mass. MSIA has dual specificity because of the antibody-antigen reaction coupled with the power of a mass spectrometer.

Nanospray desorption electrospray ionization (nano-DESI) is an ambient pressure ionization technique used in mass spectrometry (MS) for chemical analysis of organic molecules. In this technique, analytes are desorbed into a liquid bridge formed between two capillaries and the sampling surface. Unlike desorption electrospray ionization (DESI), from which nano-DESI is derived, nano-DESI makes use of a secondary capillary, which improves the sampling efficiency.
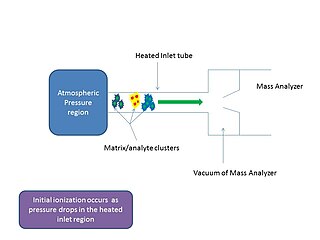
In mass spectrometry, matrix-assisted ionization is a low fragmentation (soft) ionization technique which involves the transfer of particles of the analyte and matrix sample from atmospheric pressure (AP) to the heated inlet tube connecting the AP region to the vacuum of the mass analyzer.
Probe electrospray ionization (PESI) is an electrospray-based ambient ionization technique which is coupled with mass spectrometry for sample analysis. Unlike traditional mass spectrometry ion sources which must be maintained in a vacuum, ambient ionization techniques permit sample ionization under ambient conditions, allowing for the high-throughput analysis of samples in their native state, often with minimal or no sample pre-treatment. The PESI ion source simply consists of a needle to which a high voltage is applied following sample pick-up, initiating electrospray directly from the solid needle.
Single-drop microextraction (SDME) is a sample preparation technique in chemical test or analytical chemistry. SDME uses only a single drop of solvent to isolate and preconcentrate analytes from a sample matrix. The extremely low solvent use of SDME makes it cost-effective and less harmful to the environment, subscribing to the principles of green analytical chemistry.
References
- 1 2 Richter, Bruce E.; Jones, Brian A.; Ezzell, John L.; Porter, Nathan L.; Avdalovic, Nebojsa; Pohl, Chris (1996). "Accelerated Solvent Extraction: A Technique for Sample Preparation". Anal. Chem. 68 (6): 1033–1039. doi:10.1021/ac9508199.
- ↑ Murphy, B.J.; Carlson, R.E.; Peterson, J.H.; Richter, Bruce E. (December 2, 2007). "Accelerated Solvent Extraction: Sample Preparation for Determination of Brominated Flame Retardants" . Retrieved 2016-05-09.
- ↑ "Accelerated Solvent Extraction - Dionex Solvent Extractors". analytica-world.com. Retrieved 11 September 2020.
- ↑ "Accelerated Solvent Extraction (ASE) - US". Thermo Fisher Scientific. 25 February 2019. Retrieved 11 September 2020.
- ↑ "Präanalytische Methoden". Instrumentelle Analytik und Bioanalytik. Springer-Lehrbuch (in German). Berlin, Heidelberg: Springer Berlin Heidelberg. 2008. pp. 57–90. doi:10.1007/978-3-540-73804-6_3. ISBN 978-3-540-73803-9. ISSN 0937-7433.
- ↑ Gallagher, P. A.; Shoemaker, J. A.; Wei, Xinyi; Brockhoff-Schwegel, C. A.; Creed, J. T. (8 January 2001). "Extraction and detection of arsenicals in seaweed via accelerated solvent extraction with ion chromatographic separation and ICP-MS detection". Fresenius' Journal of Analytical Chemistry. Springer Science and Business Media LLC. 369 (1): 71–80. doi:10.1007/s002160000585. ISSN 0937-0633. PMID 11210234. S2CID 46609876.
- ↑ Kellogg, Joshua J.; Wallace, Emily D.; Graf, Tyler N.; Oberlies, Nicholas H.; Cech, Nadja B. (2017). "Conventional and accelerated-solvent extractions of green tea (camellia sinensis) for metabolomics-based chemometrics". Journal of Pharmaceutical and Biomedical Analysis. Elsevier BV. 145: 604–610. doi:10.1016/j.jpba.2017.07.027. ISSN 0731-7085. PMC 5804813 . PMID 28787673.
- ↑ Cicchetti, Esmeralda; Chaintreau, Alain (2009). "Comparison of extraction techniques and modeling of accelerated solvent extraction for the authentication of natural vanilla flavors". Journal of Separation Science. Wiley. 32 (11): 1957–1964. doi:10.1002/jssc.200800650. ISSN 1615-9306. PMID 19425014.
- ↑ Shen, Jinchao; Shao, Xueguang (18 October 2005). "A comparison of accelerated solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction for analysis of terpenoids and sterols in tobacco". Analytical and Bioanalytical Chemistry. Springer Science and Business Media LLC. 383 (6): 1003–1008. doi:10.1007/s00216-005-0078-6. ISSN 1618-2642. PMID 16231136. S2CID 8169502.