Related Research Articles
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.

The Misuse of Drugs Act 1971 is an act of the Parliament of the United Kingdom. It represents action in line with treaty commitments under the Single Convention on Narcotic Drugs, the Convention on Psychotropic Substances, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

2,5-Dimethoxy-4-methylamphetamine is a psychedelic and a substituted amphetamine. It was first synthesized by Alexander Shulgin, and later reported in his book PiHKAL: A Chemical Love Story. DOM is classified as a Schedule I substance in the United States, and is similarly controlled in other parts of the world. Internationally, it is a Schedule I drug under the Convention on Psychotropic Substances. It is generally taken orally.
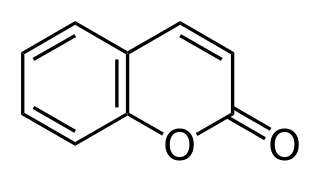
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by an unsaturated lactone ring −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and considered as a lactone.
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Glycolic acid is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate is a salt or ester of glycolic acid.
In organic chemistry, the Hammett equation describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a reaction constant. This equation was developed and published by Louis Plack Hammett in 1937 as a follow-up to qualitative observations in his 1935 publication.
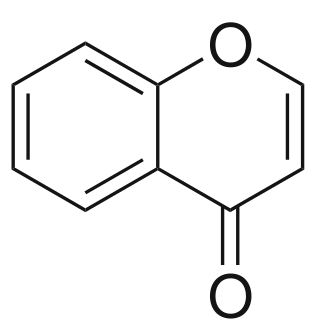
Chromone is a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin.

Acibenzolar-S-methyl is the ISO common name for an organic compound that is used as a fungicide. Unusually, it is not directly toxic to fungi but works by inducing systemic acquired resistance, the natural defence system of plants.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.

Pelanserin (TR2515) is a chemical compound that acts as an antagonist of the 5-HT2 and α1-adrenergic receptors.
The Bargellini reaction is a chemical reaction discovered in 1906 by Italian chemist Guido Bargellini. The original reaction was a mixture of the reagents phenol, chloroform, and acetone in the presence of a sodium hydroxide solution. Prior to Bargellini's research, the product attributed to this multi-component reaction (MCR) had been described as a phenol derivative in chemistry texts at the time. However, Bargellini demonstrated that a carboxylic acid derivative was actually the correct structure.
Sasanka Chandra Bhattacharyya was an Indian natural product chemist and the director of Bose Institute, Kolkata. He was known for his studies on structures and configurations of terpenoids and synthesis of Vetiver Oil and natural musk. He was the vice-president of the Indian National Science Academy and was an elected fellow of the academy as well as the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1962, for his contributions to chemical sciences.

Coumarin derivatives are derivatives of coumarin and are considered phenylpropanoids. Among the most important derivatives are the 4-hydroxycoumarins, which exhibit anticoagulant properties, a characteristic not present for coumarin itself.
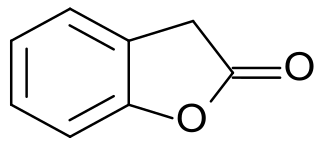
2-Cumaranone is a bicyclic heteroaromatic compound in which a six-membered benzene ring is annulated with a five-membered γ-butyrolactone ring. The 2(3H)-benzofuranone can also be considered as a lactone of (2-hydroxyphenyl)acetic acid. The benzofuranone basic structure is the basis of some natural products – such as rosmadial, which is isolatable from rosemary oil, and some substances with high pharmacological activity, such as griseofulvin and rifampicin. Furthermore, 2-cumaranone is utilized as a starting material for the preparation of chemiluminescent and fluorescent dyes, for synthetic pharmaceutical agents, like the antiarrhythmic drug dronedarone, and especially for the fungicide azoxystrobin.
References
- ↑ Adressbuch deutscher Chemiker, Verlag Chemie, 1953, p. 305
- ↑ US2805250A,Werner, Zerweck; Adolf, Stachel& Armin, Kutzsche,"Nu-nu-dibenzylsulfamyl benzoic acid",issued 1957-09-03
- ↑ "The European Library". October 2014.
- ↑ "Nu-nu-dibenzylsulfamyl benzoic acid".