
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.
Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons.
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and electrons in a molecule can be treated separately, based on the fact that the nuclei are much heavier than the electrons. Due to the larger relative mass of a nucleus compared to an electron, the coordinates of the nuclei in a system are approximated as fixed, while the coordinates of the electrons are dynamic. The approach is named after Max Born and his 23-year-old graduate student J. Robert Oppenheimer, the latter of whom proposed it in 1927 during a period of intense ferment in the development of quantum mechanics.
The van der Waals radius, rw, of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms were not simply points and to demonstrate the physical consequences of their size through the van der Waals equation of state.
Chi is the twenty-second letter of the Greek alphabet.
Slater-type orbitals (STOs) are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital method. They are named after the physicist John C. Slater, who introduced them in 1930.
In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent higher energy electrons from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. The effective nuclear charge experienced by an electron is also called the core charge. It is possible to determine the strength of the nuclear charge by the oxidation number of the atom. Most of the physical and chemical properties of the elements can be explained on the basis of electronic configuration. Consider the behavior of ionization energies in the periodic table. It is known that the magnitude of ionization potential depends upon the following factors:
- Size of atom;
- The nuclear charge;
- The screening effect of the inner shells, and
- The extent to which the outermost electron penetrates into the charge cloud set up by the inner lying electron.
In group theory, a branch of abstract algebra, a character table is a two-dimensional table whose rows correspond to irreducible representations, and whose columns correspond to conjugacy classes of group elements. The entries consist of characters, the traces of the matrices representing group elements of the column's class in the given row's group representation. In chemistry, crystallography, and spectroscopy, character tables of point groups are used to classify e.g. molecular vibrations according to their symmetry, and to predict whether a transition between two states is forbidden for symmetry reasons. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry devote a chapter to the use of symmetry group character tables.
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states, and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable.
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of particles with an electric charge. When subject to an electric field, the negatively charged electrons and positively charged atomic nuclei are subject to opposite forces and undergo charge separation. Polarizability is responsible for a material's dielectric constant and, at high (optical) frequencies, its refractive index.
In chemistry, bond energy (BE), also called the mean bond enthalpy or average bond enthalpy is a measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy for all bonds of the same type within the same chemical species.

Flory–Huggins solution theory is a lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixing. The result is an equation for the Gibbs free energy change for mixing a polymer with a solvent. Although it makes simplifying assumptions, it generates useful results for interpreting experiments.
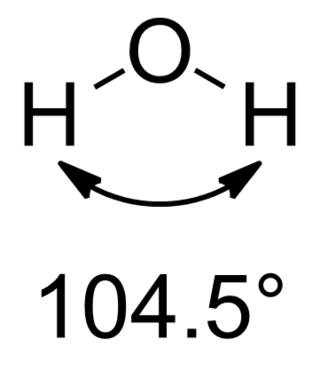
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows:
Atomic s character concentrates in orbitals directed toward electropositive substituents.
The covalent radius of fluorine is a measure of the size of a fluorine atom; it is approximated at about 60 picometres.
In mathematics, a cardinal function is a function that returns cardinal numbers.

Fluorine perchlorate, also called perchloryl hypofluorite is the rarely encountered chemical compound of fluorine, chlorine, and oxygen with the chemical formula ClO
4F or FOClO
3. It is an extremely unstable gas that explodes spontaneously and has a penetrating odor.
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or more unpaired electrons are paramagnetic. The magnitude of the paramagnetism is expressed as an effective magnetic moment, μeff. For first-row transition metals the magnitude of μeff is, to a first approximation, a simple function of the number of unpaired electrons, the spin-only formula. In general, spin–orbit coupling causes μeff to deviate from the spin-only formula. For the heavier transition metals, lanthanides and actinides, spin–orbit coupling cannot be ignored. Exchange interaction can occur in clusters and infinite lattices, resulting in ferromagnetism, antiferromagnetism or ferrimagnetism depending on the relative orientations of the individual spins.

Eugene George Rochow was an American inorganic chemist. Rochow worked on organosilicon chemistry; in the 1940s, he described the direct process, also known as the Rochow process or Müller-Rochow process.
In condensed matter and atomic physics, Van Vleck paramagnetism refers to a positive and temperature-independent contribution to the magnetic susceptibility of a material, derived from second order corrections to the Zeeman interaction. The quantum mechanical theory was developed by John Hasbrouck Van Vleck between the 1920s and the 1930s to explain the magnetic response of gaseous nitric oxide and of rare-earth salts. Alongside other magnetic effects like Paul Langevin's formulas for paramagnetism and diamagnetism, Van Vleck discovered an additional paramagnetic contribution of the same order as Langevin's diamagnetism. Van Vleck contribution is usually important for systems with one electron short of being half filled and this contribution vanishes for elements with closed shells.








