
Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d.
Phycobilins are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis.
Phycoerythrin (PE) is a red protein-pigment complex from the light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptophytes, accessory to the main chlorophyll pigments responsible for photosynthesis.The red pigment is due to the prosthetic group, phycoerythrobilin, which gives phycoerythrin its red color.

Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII.
Accessory pigments are light-absorbing compounds, found in photosynthetic organisms, that work in conjunction with chlorophyll a. They include other forms of this pigment, such as chlorophyll b in green algal and vascular ("higher") plant antennae, while other algae may contain chlorophyll c or d. In addition, there are many non-chlorophyll accessory pigments, such as carotenoids or phycobiliproteins, which also absorb light and transfer that light energy to photosystem chlorophyll. Some of these accessory pigments, in particular the carotenoids, also serve to absorb and dissipate excess light energy, or work as antioxidants. The large, physically associated group of chlorophylls and other accessory pigments is sometimes referred to as a pigment bed.

Phycocyanin is a pigment-protein complex from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble, so they cannot exist within the membrane like carotenoids can. Instead, phycobiliproteins aggregate to form clusters that adhere to the membrane called phycobilisomes. Phycocyanin is a characteristic light blue color, absorbing orange and red light, particularly near 620 nm, and emits fluorescence at about 650 nm. Allophycocyanin absorbs and emits at longer wavelengths than phycocyanin C or phycocyanin R. Phycocyanins are found in cyanobacteria. Phycobiliproteins have fluorescent properties that are used in immunoassay kits. Phycocyanin is from the Greek phyco meaning “algae” and cyanin is from the English word “cyan", which conventionally means a shade of blue-green and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue". The product phycocyanin, produced by Aphanizomenon flos-aquae and Spirulina, is for example used in the food and beverage industry as the natural coloring agent 'Lina Blue' or 'EXBERRY Shade Blue' and is found in sweets and ice cream. In addition, fluorescence detection of phycocyanin pigments in water samples is a useful method to monitor cyanobacteria biomass.
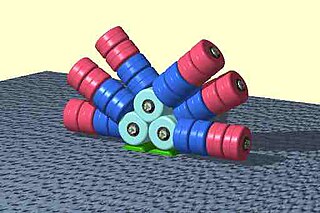
Phycobilisomes are light harvesting antennae of photosystem II in cyanobacteria, red algae and glaucophytes. It was lost in the plastids of green algae / plants (chloroplasts).

Allophycocyanin is a protein from the light-harvesting phycobiliprotein family, along with phycocyanin, phycoerythrin and phycoerythrocyanin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble and therefore cannot exist within the membrane like carotenoids, but aggregate, forming clusters that adhere to the membrane called phycobilisomes. Allophycocyanin absorbs and emits red light, and is readily found in Cyanobacteria, and red algae. Phycobilin pigments have fluorescent properties that are used in immunoassay kits. In flow cytometry, it is often abbreviated APC. To be effectively used in applications such as FACS, High-Throughput Screening (HTS) and microscopy, APC needs to be chemically cross-linked.

Phycobiliproteins are water-soluble proteins present in cyanobacteria and certain algae. They capture light energy, which is then passed on to chlorophylls during photosynthesis. Phycobiliproteins are formed of a complex between proteins and covalently bound phycobilins that act as chromophores. They are most important constituents of the phycobilisomes.

Phycoerythrobilin is a red phycobilin, i.e. an open tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Phycoerythrobilin is present in the phycobiliprotein phycoerythrin, of which it is the terminal acceptor of energy. The amount of phycoerythrobilin in phycoerythrins varies a lot, depending on the considered organism. In some Rhodophytes and oceanic cyanobacteria, phycoerythrobilin is also present in the phycocyanin, then termed R-phycocyanin. Like all phycobilins, phycoerythrobilin is covalently linked to these phycobiliproteins by a thioether bond.

A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical energy.
A light-harvesting complex consists of a number of chromophores which are complex subunit proteins that may be part of a larger super complex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. The light which is captured by the chromophores is capable of exciting molecules from their ground state to a higher energy state, known as the excited state. This excited state does not last very long and is known to be short-lived.

Biological pigments, also known simply as pigments or biochromes, are substances produced by living organisms that have a color resulting from selective color absorption. Biological pigments include plant pigments and flower pigments. Many biological structures, such as skin, eyes, feathers, fur and hair contain pigments such as melanin in specialized cells called chromatophores. In some species, pigments accrue over very long periods during an individual's lifespan.
Phycoerythrocyanin is a kind of phycobiliprotein, magenta chromoprotein involved in photosynthesis of some Cyanobacteria. This chromoprotein consists of alpha- and beta-subunits, generally aggregated as hexamer. Alpha-phycoerythrocyanin contains a phycoviolobilin, a violet bilin, that covalently attached at Cys-84, and beta-phycoerythrocyanin contains two phycocyanobilins, a blue bilin, that covalently attached at Cys-84 and -155, respectively. Phycoerythrocyanin is similar to phycocyanin, an important component of the light-harvesting complex (phycobilisome) of cyanobacteria and red algae.

Phycourobilin is an orange tetrapyrrole involved in photosynthesis in cyanobacteria and red algae. This chromophore is bound to the phycobiliprotein phycoerythrin, the distal component of the light-harvesting system of cyanobacteria and red algae (phycobilisome).
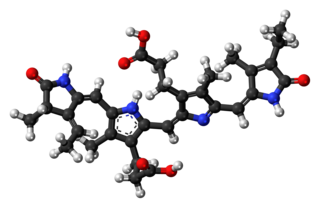
Phycocyanobilin is a blue phycobilin, i.e., a tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes, and some cryptomonads. Phycocyanobilin is present only in the phycobiliproteins allophycocyanin and phycocyanin, of which it is the terminal acceptor of energy. It is covalently linked to these phycobiliproteins by a thioether bond.

Photosynthetic reaction centre proteins are main protein components of photosynthetic reaction centres (RCs) of bacteria and plants. They are transmembrane proteins embedded in the chloroplast thylakoid or bacterial cell membrane.
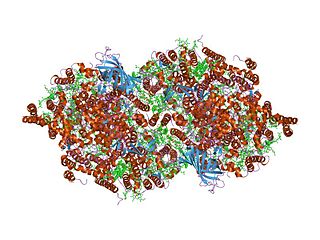
In molecular biology, the PsbZ (Ycf9) is a protein domain, which is low in molecular weight. It is a transmembrane protein and therefore is located in the thylakoid membrane of chloroplasts in cyanobacteria and plants. More specifically, it is located in Photosystem II (PSII) and in the light-harvesting complex II (LHCII). Ycf9 acts as a structural linker, that stabilises the PSII-LHCII supercomplexes. Moreover, the supercomplex fails to form in PsbZ-deficient mutants, providing further evidence to suggest Ycf9's role as a structural linker. This may be caused by a marked decrease in two LHCII antenna proteins, CP26 and CP29, found in PsbZ-deficient mutants, which result in structural changes, as well as functional modifications in PSII.
In photosynthesis, state transitions are rearrangements of the photosynthetic apparatus which occur on short time-scales. The effect is prominent in cyanobacteria, whereby the phycobilisome light-harvesting antenna complexes alter their preference for transfer of excitation energy between the two reaction centers, PS I and PS II. This shift helps to minimize photodamage caused by reactive oxygen species (ROS) under stressful conditions such as high light, but may also be used to offset imbalances between the rates of generating reductant and ATP.

Biliproteins are pigment protein compounds that are located in photosynthesising organisms such as algae and certain insects. They refer to any protein that contains a bilin chromophore. In plants and algae, the main function of biliproteins is to make the process of light accumulation required for photosynthesis more efficient; while in insects they play a role in growth and development. Some of their properties: including light-receptivity, light-harvesting and fluorescence have made them suitable for applications in bioimaging and as indicators; while other properties such as anti-oxidation, anti-aging and anti-inflammation in phycobiliproteins have given them potential for use in medicine, cosmetics and food technology. While research on biliproteins dates back as far as 1950, it was hindered due to issues regarding biliprotein structure, lack of methods available for isolating individual biliprotein components, as well as limited information on lyase reactions . Research on biliproteins has also been primarily focused on phycobiliproteins; but advances in technology and methodology, along with the discovery of different types of lyases, has renewed interest in biliprotein research, allowing new opportunities for investigating biliprotein processes such as assembly/disassembly and protein folding.











