Related Research Articles

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

The thiazolidinediones, abbreviated as TZD, also known as glitazones after the prototypical drug ciglitazone, are a class of heterocyclic compounds consisting of a five-membered C3NS ring. The term usually refers to a family of drugs used in the treatment of diabetes mellitus type 2 that were introduced in the late 1990s. As of 2024, there are two FDA-approved drugs in this class, pioglitazone and rosiglitazone.

Fortrea Holdings Inc. is a contract research organization organized in Delaware and headquartered in Durham, North Carolina with operations in 90 countries. Its customers are primarily in the pharmaceutical, biotechnology, and medical device industries.

Fatty liver disease (FLD), also known as hepatic steatosis and steatotic liver disease (SLD), is a condition where excess fat builds up in the liver. Often there are no or few symptoms. Occasionally there may be tiredness or pain in the upper right side of the abdomen. Complications may include cirrhosis, liver cancer, and esophageal varices.
In the life sciences, a contract research organization (CRO) is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biological assay development, commercialization, clinical development, clinical trials management, pharmacovigilance, outcomes research, and real world evidence.
A clinical research associate (CRA), also called a clinical monitor or trial monitor, is a health-care professional who performs many activities related to medical research, particularly clinical trials. Clinical research associates work in various settings, such as pharmaceutical companies, medical research institutes and government agencies. Depending on the jurisdiction, different education and certification requirements may be necessary, although not usually required, to practice as a clinical research associate. The main tasks of the CRA are defined by good clinical practice guidelines for monitoring clinical trials, such as those elaborated by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. A CRA would subsequently grow into a Feasibility Leader, Study Start up Leader, Project Manager, and Project Director at a Pharmaceutical company or a contract research organization. A CRA is usually required to possess an academic degree in Life Sciences and needs to have a good knowledge of good clinical practice and local regulations.
Parexel International is an American provider of biopharmaceutical services. It conducts clinical trials on behalf of its pharmaceutical clients to expedite the drug approval process. It is the second largest clinical research organization in the world and has helped develop approximately 95% of the 200 top-selling biopharmaceuticals on the market today. The company publishes the annual Parexel R&D Statistical Sourcebook and operates the Parexel-Academy.
DOKUMEDS is European Clinical Research Organization (CRO) providing a comprehensive range of services for clinical research focused primarily on Phase I-IV clinical trials and development to the pharmaceutical, biotechnology and medical device industry. Established in 1995, Dokumeds has been expanding its coverage and services significantly over 25 years of operations. Gradual geographical and operational coverage expansion has correlated with company staff growth and lowering of employee turnover. Nowadays Dokumeds is a leading international CRO with operational capabilities in 30+ countries in Europe, Africa, and other regions.
Schrödinger, Inc. is an international scientific software and biotechnology company that specializes in developing computational tools and software for drug discovery and materials science.

Cenicriviroc is an experimental drug candidate for the treatment of HIV infection and in combination with Tropifexor for non-alcoholic steatohepatitis. It is being developed by Takeda and Tobira Therapeutics.
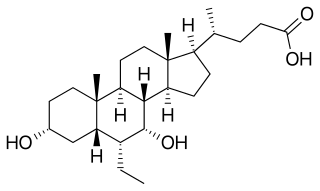
Obeticholic acid (OCA), sold under the brand name Ocaliva, is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It is used as a medication used to treat primary biliary cholangitis. Intercept Pharmaceuticals Inc. hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.
Intercept Pharmaceuticals, Inc. is an American biopharmaceutical company incorporated in 2002, focusing on the development of novel synthetic bile acid analogs to treat chronic liver diseases, such as primary biliary cirrhosis (PBC) now called primary biliary cholangitis, non-alcoholic fatty liver disease, cirrhosis, portal hypertension, primary sclerosing cholangitis and also the intestinal disorder, bile acid diarrhea.

Arcturus Therapeutics Holdings Inc. is an American RNA medicines biotechnology company focused on the discovery, development and commercialization of therapeutics for rare diseases and infectious diseases. Arcturus has developed proprietary lipid nanoparticle RNA therapeutics for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides, and microRNA.

Emricasan is a potential drug invented in 1998 by Idun Pharmaceuticals. The drug was acquired by Pfizer in 2005 and then sold to Conatus Pharmaceuticals in 2010. Conatus in turn licensed emricasan to Novartis in 2017 for exclusive development and commercialization.

Melissa Palmer is an American hepatologist. She is recognized for her research and treatment of hepatitis and liver disease. Palmer is the Chief Medical Officer of Gannex Pharma, a wholly owned company of Ascletis Pharma.
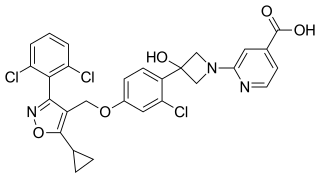
Cilofexor is a nonsteroidal farnesoid X receptor (FXR) agonist in clinical trials for the treatment of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and primary sclerosing cholangitis (PSC). It is being investigated for use alone or in combination with firsocostat, selonsertib, or semaglutide. In rat models and human clinical trials of NASH it has been shown to reduce fibrosis and steatosis, and in human clinical trials of PSC it improved cholestasis and reduced markers of liver injury.
BIOTECanada, previously the Industrial Biotechnology Association of Canada, is a Canadian biotechnology industry association based in Ottawa, Ontario. It is an industry-funded membership organization composed of over 250 national and international pharmaceutical and gene therapy companies, medical device manufacturers, agricultural science businesses, law firms, academic institutions, research and development networks, advertising agencies, insurance companies and financial services firms.
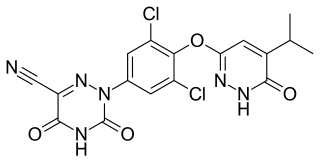
Resmetirom, sold under the brand name Rezdiffra, is a medication used for the treatment of noncirrhotic nonalcoholic steatohepatitis. It is a thyroid hormone receptor beta (NR1A2) agonist.
Belapectin is a galectin-3 inhibitor developed by Galectin Therapeutics that failed phase 3 clinical trials for the treatment of non-alcoholic steatohepatitis.

HU6 is the first controlled metabolic accelerator. This class of drugs is designed to increase basal metabolic rate by a small, imperceptible amount. The result is a selective increase in fat metabolism and loss of body fat, especially visceral fat. There is also an independent reduction in reactive oxygen species or free radicals, thus reducing systemic inflammation. Developed by Rivus Pharmaceuticals, the drug is tested to reduce weight and liver fat in humans with risk factors for metabolic dysfunction-associated steatohepatitis. In a phase 2a trial, the higher dosage levels reduced liver fat on average by more than 30 percent and also reduced body weight significantly. A phase 2b trial in patients with metabolic dysfunction-associated steatohepatitis was subsequently initiated. Data from this study are expected to be reported in 2025. Additionally, a phase 2a study was performed in patients suffering from heart failure with preserved ejection fraction, a disease that is mediated by visceral fat and obesity. The study achieved the primary endpoint of weight loss, as well as a number of secondary endpoints.
References
- ↑ Alimenitv. "Precision Medicine" . Retrieved 30 April 2024.
- ↑ Alimentiv. "Statistics" . Retrieved 30 April 2024.
- ↑ Alimentiv Inc. "Medical R&D" . Retrieved 18 April 2023.
- ↑ PR Newswire. "Alimentiv, Satisfai Health, and Virgo Announce Partnership to use AI-driven technology to Enhance Clinical Trials in IBD and other GI Diseases" . Retrieved 18 April 2023.
- ↑ London Inc. Magazine. "Robarts Clinical Trials rebrands as Alimentiv Inc" . Retrieved 18 April 2023.
- ↑ De Bono, Norman. "Growth prompts new name for London health-care business". The London Free Press. Retrieved 30 April 2024.
- ↑ Cision. "Alimentiv, Summit Clinical Research Announce Collaboration to drive Non-Alcoholic Steatohepatitis (NASH) Clinical Trials" . Retrieved 30 April 2024.
- ↑ Cision (3 November 2022). "Alimentiv, Summit Clinical Research Announce Collaboration to drive Non-Alcoholic Steatohepatitis (NASH) Clinical Trials" . Retrieved 18 April 2023.
- ↑ Brooks, Kristin (2 March 2021). "Alimentiv Acquires McDougall Scientific" . Retrieved 18 April 2023.
- ↑ Cision (14 April 2021). "Alimentiv Health Trust Launches AcelaBio (US) Inc. - A Commercial State-of-the-art Research Laboratory Delivering Histopathology And Precision Medicine Services For Global Clinical Trials" . Retrieved 18 April 2023.