
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.
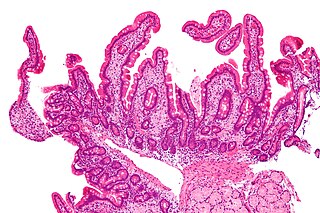
Whipple's disease is a rare systemic infectious disease caused by the bacterium Tropheryma whipplei. First described by George Hoyt Whipple in 1907 and commonly considered as a gastrointestinal disorder, Whipple's disease primarily causes malabsorption, but may affect any part of the human body, including the heart, brain, joints, skin, lungs and the eyes. Weight loss, diarrhea, joint pain, and arthritis are common presenting symptoms, but the presentation can be highly variable in certain individuals, and about 15% of patients do not have the standard signs and symptoms.

An asymptomatic carrier is a person or other organism that has become infected with a pathogen, but shows no signs or symptoms.
HIV-associated neurocognitive disorders (HAND) are neurological disorders associated with HIV infection and AIDS. It is a syndrome of progressive deterioration of memory, cognition, behavior, and motor function in HIV-infected individuals during the late stages of the disease, when immunodeficiency is severe. HAND may include neurological disorders of various severity. HIV-associated neurocognitive disorders are associated with a metabolic encephalopathy induced by HIV infection and fueled by immune activation of macrophages and microglia. These cells are actively infected with HIV and secrete neurotoxins of both host and viral origin. The essential features of HIV-associated dementia (HAD) are disabling cognitive impairment accompanied by motor dysfunction, speech problems and behavioral change. Cognitive impairment is characterised by mental slowness, trouble with memory and poor concentration. Motor symptoms include a loss of fine motor control leading to clumsiness, poor balance and tremors. Behavioral changes may include apathy, lethargy and diminished emotional responses and spontaneity. Histopathologically, it is identified by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes and neuronal loss.

Meningoencephalitis, also known as herpes meningoencephalitis, is a medical condition that simultaneously resembles both meningitis, which is an infection or inflammation of the meninges, and encephalitis, which is an infection or inflammation of the brain tissue.
Sulfatide, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebroside, is a class of sulfolipids, specifically a class of sulfoglycolipids, which are glycolipids that contain a sulfate group. Sulfatide is synthesized primarily starting in the endoplasmic reticulum and ending in the Golgi apparatus where ceramide is converted to galactocerebroside and later sulfated to make sulfatide. Of all of the galactolipids that are found in the myelin sheath, one fifth of them are sulfatide. Sulfatide is primarily found on the extracellular leaflet of the myelin plasma membrane produced by the oligodendrocytes in the central nervous system and in the Schwann cells in the peripheral nervous system. However, sulfatide is also present on the extracellular leaflet of the plasma membrane of many cells in eukaryotic organisms.
Host tropism is the infection specificity of certain pathogens to particular hosts and host tissues. This explains why most pathogens are only capable of infecting a limited range of host organisms.
Visna-maedi virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as ovine progressive pneumonia (OPP), particularly in the United States, or Montana sheep disease. White blood cells of the monocyte/macrophage lineage are the main target of the virus.

Janice Ellen Clements is vice dean for faculty at the Johns Hopkins School of Medicine and the Mary Wallace Stanton Professor of Faculty Affairs. She is a professor in the departments of Molecular and Comparative Pathobiology, Neurology, and Pathology, and has a joint appointment in molecular biology and genetics. Her molecular biology and virology research examines lentiviruses and how they cause neurological diseases.
M. Christine "Chris" Zink is the director of the Department of Molecular and Comparative Pathobiology at the Johns Hopkins School of Medicine. She also holds professorships in the Department of Pathology at Johns Hopkins and in the Department of Molecular Microbiology and Immunology at the Johns Hopkins Bloomberg School of Public Health. Zink researches the response of the immune system to retroviruses such as HIV and is currently investigating an animal model of antiretroviral therapy and the potential of a common antibiotic to prevent HIV-associated neurocognitive disorders.
Neurovirology is an interdisciplinary field which represents a melding of clinical neuroscience, virology, immunology, and molecular biology. The main focus of the field is to study viruses capable of infecting the nervous system. In addition to this, the field studies the use of viruses to trace neuroanatomical pathways, for gene therapy, and to eliminate detrimental populations of neural cells.

Gladstone Institutes is an independent, non-profit biomedical research organization whose focus is to better understand, prevent, treat and cure cardiovascular, viral and neurological conditions such as heart failure, HIV/AIDS and Alzheimer's disease. Its researchers study these diseases using techniques of basic and translational science. Another focus at Gladstone is building on the development of induced pluripotent stem cell technology by one of its investigators, 2012 Nobel Laureate Shinya Yamanaka, to improve drug discovery, personalized medicine and tissue regeneration.

The stages of HIV infection are acute infection, latency, and AIDS. Acute infection lasts for several weeks and may include symptoms such as fever, swollen lymph nodes, inflammation of the throat, rash, muscle pain, malaise, and mouth and esophageal sores. The latency stage involves few or no symptoms and can last anywhere from two weeks to twenty years or more, depending on the individual. AIDS, the final stage of HIV infection, is defined by low CD4+ T cell counts, various opportunistic infections, cancers, and other conditions.

Lalita Ramakrishnan is an Indian-born American microbiologist who is known for her contributions to the understanding of the biological mechanism of tuberculosis. As of 2019 she serves as a professor of Immunology and Infectious Diseases at the University of Cambridge, where she is also a Wellcome Trust Principal Research Fellow and a practicing physician. Her research is conducted at the MRC Laboratory of Molecular Biology, where she serves as the Head of the Molecular Immunity Unit of the Department of Medicine embedded at the MRC LMB. Working with Stanley Falkow at Stanford, she developed the strategy of using Mycobacterium marinum infection as a model for tuberculosis. Her work has appeared in a number of journals, including Science, Nature, and Cell. In 2018 and 2019 Ramakrishnan coauthored two influential papers in the British Medical Journal (BMJ) arguing that the widely accepted estimates of the prevalence of latent tuberculosis—estimates used as a basis for allocation of research funds—are far too high. She is married to Mark Troll, a physical chemist.

Henry Charles Mwandumba is an African Professor of Medicine and the Director of the Malawi-Liverpool-Wellcome Programme. He works on the tuberculosis phagosome in the University of Malawi College of Medicine, and serves as President of the Federation of African Immunological Societies. In 2019 Mwandumba was awarded the Royal Society Africa Prize.
Ya-Chi Ho is a Taiwanese infectious disease researcher and Associate Professor of Microbial Pathogenesis and Medicine at Yale University. Her research centers on the interaction between HIV and the host's immune system with the ultimate goal of curing HIV/AIDS.
Georgette D. Kanmogne is a Cameroonian American geneticist and molecular virologist and a full professor and vice chair for resource allocation and faculty development within the Department of Pharmacology and Experimental Neurosciences at the University of Nebraska Medical Center in Omaha, Nebraska. Kanmogne's research program focuses on exploring the pathogenesis of neuroAIDS by deciphering the mechanisms underlying blood brain barrier dysfunction and viral entry into the central nervous system. Her research also addresses the lack of HIV therapies that cross the blood brain barrier (BBB) and has played a critical role in the development of nanoparticles encapsulating HIV-drugs that can cross the BBB to prevent viral-mediated neuron death in the brain. Kanmogne collaborates with clinical and basic researchers across America, Cameroon, and West Africa, spanning disciplines from hematology to psychiatry, to explore how viral genetic diversity is correlated with the neurological impact of HIV.
Crystal C. Watkins Johansson is an American neuroscientist and psychiatrist and associate professor of neuroscience at Johns Hopkins University School of Medicine as well as the director of the Sheppard Pratt Memory Clinic in Neuropsychiatry in Baltimore, Maryland. Johansson was the first Black female Meyerhoff Scholar to obtain an MD/PhD from the University of Maryland, Baltimore County. During her MD/PhD she developed a novel treatment for gastrointestinal in patients with diabetes that led to a patent for a pharmacological compound in 2000. Johansson is a practicing neuropsychiatrist with a focus on geriatric psychiatry and she conducts brain imaging research as well as research on cancer in African American women.
Howard E. Gendelman is an American physician-scientist whose research intersects the disciplines of neuroimmunology, pharmacology, and infectious diseases. Gendelman was born in Philadelphia, Pennsylvania. His research is focused on harnessing immune responses for therapeutic gain in HIV/AIDS and Neurodegenerative disease. He is the Margaret R. Larson Professor of infectious diseases and internal medicine at the University of Nebraska Medical Center (UNMC) in Omaha.
Justin C. McArthur is an UK-born American neurologist. He currently holds the John W. Griffin Professorship and serves as the Chair/Director of Neurology at the Johns Hopkins University School of Medicine. He also holds the position of neurologist-in-chief at the Johns Hopkins Hospital. Additionally, he founded and led the Sheikh Khalifa Stroke Institute at Johns Hopkins.









