This article needs more reliable medical references for verification or relies too heavily on primary sources .(April 2017) |  |
Amine fluorides are dental drugs.
This article needs more reliable medical references for verification or relies too heavily on primary sources .(April 2017) |  |
Amine fluorides are dental drugs.
Amine fluorides were developed in the 1950s by GABA in collaboration with the Institute of Dentistry of the University of Zurich (Switzerland).
For the first time, in 1954, Wainwright showed in his study the high permeability of tooth enamel to organic molecules like urea. This aspect made him ask himself if it was not possible to enrich the contents of the enamel with fluoride by using organic molecules as carrier, which were chemically bonded to amino fluoride. [1]
In 1957, Mühlemann, Schmid and König published the results of their studies in vitro, in which they demonstrated that some compounds with amino fluoride were obviously superior to those with inorganic fluoride in reducing the solubility of the enamel. [2]
In the same year, Irwin, Leaver and Walsh published the results of their experiments in vitro, which demonstrated that monoamine-aliphatic compounds offered protection to the enamel against acid decalcification. [3]
In 1967, Muhleman demonstrated the superiority of organic fluoride compared to inorganic fluoride in preventing dental decay. He observed that amine fluoride had a pronounced affinity regarding enamel, by raising the quantity of fluoride in the enamel and also having an antienzyme effect on the microbial activity of dental plaque. His conclusions were the following:
In this way amine fluorides were born in GABA S.A.-BASEL laboratory.
The commercial products, which contain amine fluoride or compounds of this with tin-fluoride in their formula, are present under different forms: - gels, - fluids, - dentifrice, - mouth rinse.
The unique position of the amine fluoride is based on their special molecular structure: the fluoride ion is bound to an organic fatty acid amine fragment. This is not the case for the inorganics fluorides such as sodium fluoride and sodium monofluorophosphate.
Amine fluorides have a hydrophobic molecular moiety, the non-polar tail, with a hydrophilic component, the polar amine head. For this reason, they act like surfactants, reducing the surface tension of saliva, and forming a homogeneous film on all oral surfaces.
Due to their surface activity the amine fluorides are rapidly dispersed in the oral cavity and wet all surfaces. In contrast, in the case of inorganic fluorides the counter ion (e.g. sodium) has no transport function; the fluoride is statistically distributed in the oral cavity. Amine fluoride covers the tooth surfaces with a homogeneous molecular layer. This continuous film prevents rapid rinsing off by the saliva. The amine fluorides are thus available as an active agent for a longer period.
Amine fluorides have a slightly acidic pH. For this reason, fluoride ions can combine rapidly with the calcium in dental enamel to form calcium fluoride. This acts as a fluoride depot over a longer period: Under cariogenic conditions fluoride ions are made available stimulating the remineralisation of dental enamel and thus prevent acid attacks.
Fluoride is an inorganic, monatomic anion of fluorine, with the chemical formula F−
, whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin.

Saliva is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells, enzymes, and antimicrobial agents.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48-52%) but there are also stronger solutions and pure HF has a boiling point near room temperature. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.

Tooth enamel is one of the four major tissues that make up the tooth in humans and many animals, including some species of fish. It makes up the normally visible part of the tooth, covering the crown. The other major tissues are dentin, cementum, and dental pulp. It is a very hard, white to off-white, highly mineralised substance that acts as a barrier to protect the tooth but can become susceptible to degradation, especially by acids from food and drink. In rare circumstances enamel fails to form, leaving the underlying dentin exposed on the surface.

Tooth decay, also known as cavities or caries, is the breakdown of teeth due to acids produced by bacteria. The cavities may be a number of different colors, from yellow to black. Symptoms may include pain and difficulty eating. Complications may include inflammation of the tissue around the tooth, tooth loss and infection or abscess formation. Tooth regeneration is an ongoing stem cell–based field of study that aims to find methods to reverse the effects of decay; current methods are based on easing symptoms.

Uremia is the condition of having high levels of urea in the blood. Urea is one of the primary components of urine. It can be defined as an excess in the blood of amino acid and protein metabolism end products, such as urea and creatinine, which would normally be excreted in the urine. Uremic syndrome can be defined as the terminal clinical manifestation of kidney failure. It is the signs, symptoms and results from laboratory tests which result from inadequate excretory, regulatory, and endocrine function of the kidneys. Both uremia and uremic syndrome have been used interchangeably to denote a very high plasma urea concentration that is the result of renal failure. The former denotation will be used for the rest of the article.

Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in contrast to molecular hydrides such as borane, silane, germane, ammonia, and methane. It is an ionic material that is insoluble in all solvents (other than molten sodium metal), consistent with the fact that H− ions do not exist in solution.
Tooth whitening or tooth bleaching is the process of lightening the colour of human teeth. Whitening is often desirable when teeth become yellowed over time for a number of reasons, and can be achieved by changing the intrinsic or extrinsic colour of the tooth enamel. The chemical degradation of the chromogens within or on the tooth is termed as bleaching.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2022, it was the 221st most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.

Tetrasodium pyrophosphate, also called sodium pyrophosphate, tetrasodium phosphate or TSPP, is an inorganic compound with the formula Na4P2O7. As a salt, it is a white, water-soluble solid. It is composed of pyrophosphate anion and sodium ions. Toxicity is approximately twice that of table salt when ingested orally. Also known is the decahydrate Na4P2O7 · 10(H2O).

Fluoride therapy is the use of fluoride for medical purposes. Fluoride supplements are recommended to prevent tooth decay in children older than six months in areas where the drinking water is low in fluoride. It is typically used as a liquid, pill, or paste by mouth. Fluoride has also been used to treat a number of bone diseases.

Sodium monofluorophosphate, commonly abbreviated SMFP, is an inorganic compound with the chemical formula Na2PO3F. Typical for a salt, SMFP is odourless, colourless, and water-soluble. This salt is an ingredient in some toothpastes.

Tin(II) fluoride, commonly referred to commercially as stannous fluoride (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
Olaflur is a fluoride-containing substance that is an ingredient of toothpastes and solutions for the prevention of dental caries. It has been in use since 1966. Especially in combination with dectaflur, it is also used in the form of gels for the treatment of early stages of caries, sensitive teeth, and by dentists for the refluoridation of damaged tooth enamel.
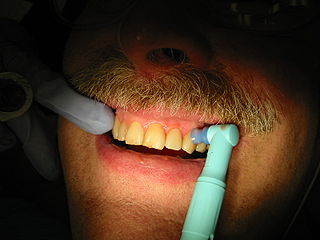
Tooth polishing procedures are done to smooth the surfaces of teeth and restorations. The purpose of polishing is to remove extrinsic stains, remove dental plaque accumulation, increase aesthetics and to reduce corrosion of metallic restorations. Tooth polishing has little therapeutic value and is usually done as a cosmetic procedure after debridement and before fluoride application. Common practice is to use a prophy cup—a small motorized rubber cup—along with an abrasive polishing compound.

Fluoride varnish is a highly concentrated form of fluoride that is applied to the tooth's surface by a dentist, dental hygienist or other dental professional, as a type of topical fluoride therapy. It is not a permanent varnish but due to its adherent nature it is able to stay in contact with the tooth surface for several hours. It may be applied to the enamel, dentine or cementum of the tooth and can be used to help prevent decay, remineralise the tooth surface and to treat dentine hypersensitivity. There are more than 30 fluoride-containing varnish products on the market today, and they have varying compositions and delivery systems. These compositional differences lead to widely variable pharmacokinetics, the effects of which remain largely untested clinically.

Tooth remineralization is the natural repair process for non-cavitated tooth lesions, in which calcium, phosphate and sometimes fluoride ions are deposited into crystal voids in demineralised enamel. Remineralization can contribute towards restoring strength and function within tooth structure.
Hans Rudolf Mühlemann was a Swiss dentist and medical academic. He was professor and director of the Dental Instituts University of Zurich.
Topical fluorides are fluoride-containing drugs indicated in prevention and treatment of dental caries, particularly in children's primary dentitions. The dental-protecting property of topical fluoride can be attributed to multiple mechanisms of action, including the promotion of remineralization of decalcified enamel, the inhibition of the cariogenic microbial metabolism in dental plaque and the increase of tooth resistance to acid dissolution. Topical fluoride is available in a variety of dose forms, for example, toothpaste, mouth rinses, varnish and silver diamine solution. These dosage forms possess different absorption mechanisms and consist of different active ingredients. Common active ingredients include sodium fluoride, stannous fluoride, silver diamine fluoride. These ingredients account for different pharmacokinetic profiles, thereby having varied dosing regimes and therapeutic effects. A minority of individuals may experience certain adverse effects, including dermatological irritation, hypersensitivity reactions, neurotoxicity and dental fluorosis. In severe cases, fluoride overdose may lead to acute toxicity. While topical fluoride is effective in preventing dental caries, it should be used with caution in specific situations to avoid undesired side effects.

Artificial saliva or salivary substitutes refer to a synthetically produced liquid that mimics the natural secretion of saliva. It is designed as a symptomatic relief for xerostomia, a condition characterised by dryness in the mouth and is available over-the-counter. The efficacy of artificial saliva in a systematic review of clinical trials indicates that all evaluated products reduce xerostomia symptoms, but the comparative effectiveness remains unclear due to study inconsistencies and potential biases. Side effects are uncommon, but users should take precautions against possible side effects such as allergic reactions.